
|
시장보고서
상품코드
1771305
의약품 의료기기 조합 제품 시장 : 업계 동향 및 예측 - 기기 유형별, 서비스 중점 분야별, 기업 규모별, 주요 지역별Drug Device Combination Products Market: Industry Trends and Global Forecasts - Distribution by Type of Device, Service Focus Area, Company Size, and Key Geographical Regions |
||||||
세계의 의약품 의료기기 조합 제품 시장 : 성장 및 동향
처방된 약물 요법에 대한 논애드히어런스(복약 미준수)는 환자의 건강에 악영향을 미칠 뿐만 아니라 제약 업계 전체의 부담을 증대시키는 중대한 문제입니다. Contract Pharma가 최근 보고한 바와 같이 복약 미준수는 의약품 개발 기업에 있어 매년 2,500억 달러 이상의 비용이 될 것으로 추정되고 있습니다. 게다가 원약(API)을 표적 전달 부위에서 해방 또는 제어 방출하기 위한 효과적인 솔루션이 없는 것은 약물 전달에 있어서 큰 과제로 남아 있습니다. 실제로 API 방출 제어가 미흡하면 혈장 내 약물 농도가 변동돼 다양한 부작용을 일으킬 수 있습니다. 이 때문에 환자를 위한 효과적인 약물 전달 치료 옵션에 대한 수요가 높아지고 있습니다.
특필해야할 것은 최근 조합 제품의 개발이 제약 회사로부터 큰 관심을 모으고 있는 것입니다. 이 제품은 FDA가 규제하는 2개 이상의 성분을 통합해 원약의 제어 방출을 가능하게 하는 것입니다. 그러나 의약품 의료기기의 조합 제품을 개발하는 것은 혁신적인 기술, 많은 투자, 전문적인 지식을 필요로 하는 복잡하고 시간이 걸리는 프로세스입니다. 추가 과제로는 의약품과 의료기기의 적합성 확보, 사용자의 요구사항에 대한 대응, 방출 시험 실시, 제조 및 포장 기준 준수 등을 들 수 있습니다. 이러한 허들에 대처해, 환자의 전귀를 개선하기 위해, 많은 개발 기업은 현재, 조합 제품의 시험 및 검증 서비스를 제공하는 전문 기업에 주된 업무를 아웃소싱하고 있습니다.
세계의 의약품 의료기기 조합 제품 시장 : 주요 인사이트
본 보고서에서는 세계 의약품 의료기기 조합 제품 시장의 현상을 조사했으며, 잠재적인 성장 기회를 특정하고 있습니다. 주요 조사 결과는 다음과 같습니다.
- 현재 세계 25개 이상의 기업이 다양한 의약품 의료기기의 조합 제품 개발 및 시험 서비스를 제공합니다.
- 서비스 제공업체는 다양한 유형의 조합 제품에 광범위한 서비스를 제공하는 데 필요한 전문 지식을 가지고 있다고 주장합니다.
- 약물 전달 기기 및 시스템의 개발과 시험을 서포트하는 기업의 대부분(85% 이상)은 북미에 본사를 두고 있습니다.
- 45% 이상의 서비스 제공업체가 조합 제품의 성능 시험과 제품 개발을 지원하고 있으며, 그 중 40%는 대기업 및 초대형입니다.
- 조합 제품 서비스 제공업체의 약 75%는 프로세스 개발과 밸리데이션을 실시하고 있어 BS, EKG Labs, Exponent, Surpass 등이 그 예입니다.
- 이 경쟁이 치열한 업계에서 우위를 차지하기 위해서, 서비스 제공업체는 기존의 서비스 내용을 업그레이드 하는 대처를 계속적으로 실시하고 있습니다.
- 증가하는 수요에 대응하기 위해 많은 기업이 서비스 포트폴리오를 강화하기 위해 신규 시설의 설립(-40%) 및 기존 능력의 확대(-50%) 등의 확대책을 실시했습니다.
- 확장 이니셔티브의 대부분은 미국을 거점으로 하는 조합 제품 서비스 제공업체별로 실시된 것으로, DDL, NAMSA, Nelson Labs 등이 그 예입니다.
- 대부분의 확장은 조합 제품과 관련된 검사 서비스에 대한 수요 증가에 대응하기 위해 실시되었습니다.
- 보고된 확장의 대부분은 지역에서의 노력이었지만, 미국을 거점으로 하는 일부 기업은 국제적인 확장의 노력도 하고 있습니다.
- 현재 210개가 넘는 조합 제품이 다양한 임상 증상의 치료용으로 개발되고 있으며, 현재 개발 중입니다. 이러한 조합 제품은 비경구 및 비경구 약물 전달 모두에 사용할 수 있습니다.
- 파이프라인에는 다양한 치료 영역용으로 개발되었거나 개발 중인 다양한 조합 제품이 있으며, 그 대부분은 생물제제의 투여용으로 평가되고 있습니다.
- 현재 개발 중인 대용량 WI 조합 제품의 대부분(약 80%)은 기초 용량을 제공하기 위해 개발되고 있습니다.
- 대용량 WI 조합 제품의 상당한 비율(33%)이 신경질환의 치료용으로 개발되고 있으며, 예로서 D-mine(R) Pump, ND0701을 들 수 있습니다.
- 프리필드 시린지 조합 제품의 90% 이상이 생물 제제 투여용으로 개발되었으며, 현재 개발중입니다.
- 조합 제품 개발 기업은 독자적인 조합 제품의 개발과 시험을 위해서, 서비스 제공업체와 전략적 제휴를 맺을 것으로 예측됩니다.
- 당사는 지적 자본을 바탕으로 개발자가 제조를 외주할지 자사에서 할지를 결정할 수 있는 독자적인 프레임워크를 제안하고 있습니다.
- 조합 제품 수요가 높아짐에 따라, 서비스 제공업체의 관련 시장은 향후 10년간, 연율 환산으로 안정된 성장이 전망됩니다.
의약품 의료기기 조합 제품 시장에서 진출기업 예
- EKG LABS
- Eurofins Medical Device Testing
- Exponent
- Kymanox
- Medical Engineering Technologies
- Pace Analytical Services
- Suttons Creek
- West Pharmaceutical Services
본 보고서에서는 세계의 의약품 의료기기 조합 제품 시장에 대해 조사했으며, 시장 개요와 함께, 기기 유형별, 서비스 중점 분야별, 기업 규모별 동향, 지역별 동향, 시장 진출기업 프로파일 등의 정보를 제공합니다.
목차
제1장 서문
제2장 주요 요약
제3장 서문
- 장의 개요
- 조합 제품 서문
- 조합 제품의 개발에 따른 과제
- 아웃소싱된 조합 제품 업무 및 서비스
- 조합 제품의 아웃소싱 업무의 장점
- CDMO를 선택할 때 고려해야 할 중요한 점
- 장래의 전망
제4장 조합 제품 서비스 제공업체 : 시장 상황
- 장의 개요
- 조합 제품 서비스 제공업체 : 시장 상황
제5장 기업 경쟁력 분석
- 장의 개요
- 전제 및 주요 파라미터
- 조사 방법
- 조합 제품 서비스 제공업체 : 기업 경쟁력 분석
제6장 조합 제품 서비스 제공업체 : 기업 프로파일
- 장의 개요
- EKG LABS
- Eurofins Medical Device Testing
- Exponent
- Kymanox
- Medical Engineering Technologies
- Pace Analytical Services
- Suttons Creek
- West Pharmaceutical Services
- 결론 : 업계 진출기업이 실시하는 대처
제7장 최근 확장
제8장 케이스 스터디 : 대용량 웨어러블 인젝터 및 프리필드 시린지의 조합 제품
- 장의 개요
- 대용량 웨어러블 인젝터 조합 제품
- 프리필드 주사기 조합 제품
제9장 잠재적인 파트너 분석
- 장의 개요
- 대용량 웨어러블 인젝터 조합 제품
- 프리필드 주사기 조합 제품
제10장 아웃소싱 : GO/NO-GO 프레임워크
- 장의 개요
- 아웃소싱 : Go/No-Go 프레임워크
- 프리필드 시린지 조합 제품 개발자 : Go/No-Go 프레임워크
제11장 시장 예측
- 장의 개요
- 주요 전제 및 예측 조사 방법
- 세계의 조합 제품 서비스 제공업체 시장(-2035년)
- 세계의 조합 제품 서비스 제공업체 시장 : 기기 유형별
- 세계의 조합 제품 서비스 제공업체 시장 : 서비스 중점 분야별
- 세계의 조합 제품 서비스 제공업체 시장 : 기업 규모별
- 세계의 조합 제품 서비스 제공업체 시장 : 지역별
제12장 결론
제13장 주요 인사이트
제14장 부록 1 : 표 형식 데이터
제15장 부록 2 : 기업 및 단체 일람
AJY 25.07.21GLOBAL DRUG DEVICE COMBINATION PRODUCTS MARKET: GROWTH AND TRENDS
Non-adherence to prescribed medication regimens is a significant issue that not only negatively impacts patient health but also increases the overall burden on the pharmaceutical industry. As recently reported by Contract Pharma, medication non-adherence is estimated to cost drug developers over USD 250 billion each year. Additionally, the lack of effective solutions for sustained or controlled release of active pharmaceutical ingredients (APIs) at targeted delivery sites remains a major challenge in drug delivery. In fact, inadequate control over API release can lead to fluctuations in plasma drug levels, which may result in various side effects. This has led to a rise in the demand for effective drug delivery treatment options for patients.
Notably, in the recent past the development of combination products has attracted significant interest from pharmaceutical companies. These products integrate two or more FDA-regulated components to enable controlled release of APIs. However, creating drug-device combination products is a complex and lengthy process that demands innovative technologies, substantial investment, and specialized expertise. Additional challenges include ensuring drug-device compatibility, addressing user requirements, conducting release testing, and meeting production and packaging standards. To address these hurdles and improve patient outcomes, many developers are now outsourcing key operations to specialized companies that provide testing and validation services for combination products.
GLOBAL DRUG DEVICE COMBINATION PRODUCTS MARKET: KEY INSIGHTS
The report delves into the current state of global drug device combination products market and identifies potential growth opportunities within industry. Some key findings from the report include:
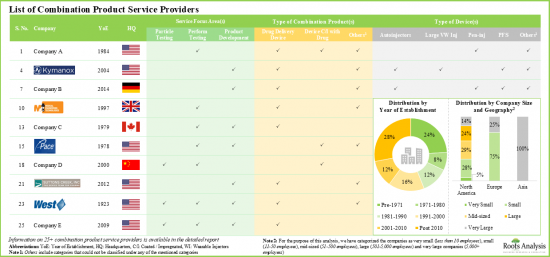
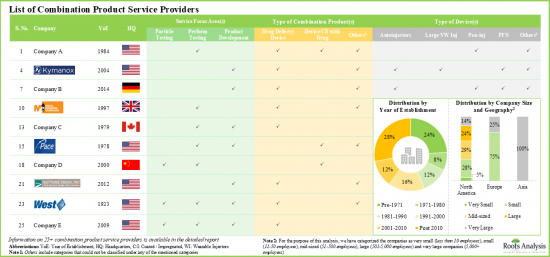
- At present, more than 25 companies across the globe offer services for the development and testing of various drug-device combination products.
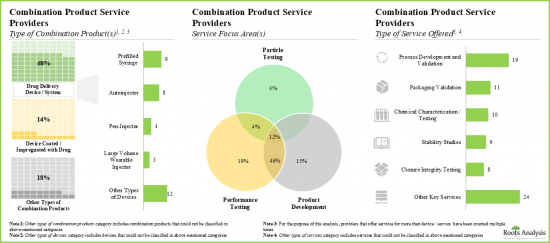
- Service providers claim to have the necessary expertise to provide a wide range of services for different types of combination products; majority (~20%) of these services are focused on performance testing.
- Majority of the players (over 85%) that support development and testing of drug delivery devices / systems are headquartered in North America.
- Over 45% of the service providers assist the performance testing and product development of combination products; of these, 40% are large / very large players.
- Around 75% of the combination product service providers perform the process development and validation; examples include BS, EKG Labs, Exponent and Surpass.
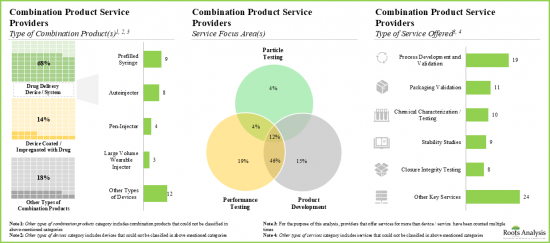
- In order to gain an edge in this competitive industry, service providers are continuously undertaking initiatives to upgrade their existing service offerings.
- To keep pace with the growing demand, many companies have undertaken expansion initiatives, such as establishing new facilities (~40%) or expanding their existing capabilities (~50%), to strengthen their service portfolio.
- Majority of the expansion initiatives were undertaken by combination product service providers based in the US; examples include DDL, NAMSA, and Nelson Labs.
- Majority expansions were undertaken in order to meet the increasing demand for testing services related to combination products.
- Most of the expansions reported were local initiatives; some companies based in the US have undertaken international expansions initiatives as well.
- Presently, over 210 combination products have been / are being developed for the treatment of various clinical conditions; these can be used for both parenteral as well as non-parenteral drug delivery.
- The pipeline features a variety of combination products that have been / are being developed for various therapeutic areas; majority of the products are being evaluated for the administration of biologics.
- Majority of the large volume WI combination products (~80%) that are currently under development are being developed to provide basal dose.
- A sizeable proportion of large volume WI combination products are being developed for the treatment of neurological disorders (33%); examples include D-mine(R) Pump, and ND0701.
- Over 90% of the prefilled syringe combination products have been / are being developed to administer biologics; of these, majority are antibody-based molecules (48%).
- Combination product developers are anticipated to forge strategic alliances with service providers for the development and testing of their proprietary combination products.
- Built on our intellectual capital, we have proposed a proprietary framework to allow developers to decide whether to outsource manufacturing or keep it in-house; we expect majority of the small firms to outsource such operations.
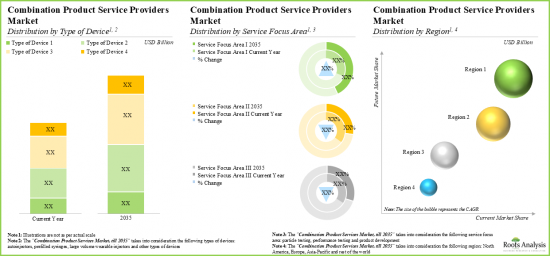
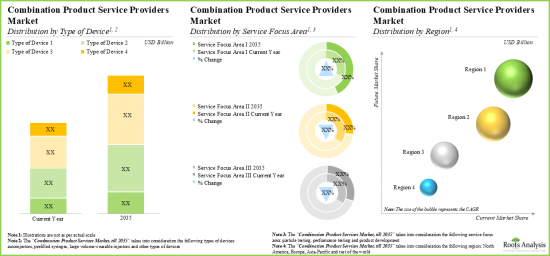
- With the rising demand for combination products, the affiliated market for service providers is expected to grow at a steady annualized rate over the coming decade.
Example Players in the Drug Device Combination Products Market
- EKG LABS
- Eurofins Medical Device Testing
- Exponent
- Kymanox
- Medical Engineering Technologies
- Pace Analytical Services
- Suttons Creek
- West Pharmaceutical Services
GLOBAL DRUG DEVICE COMBINATION PRODUCTS MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global drug device combination products market, focusing on key market segments, including [A] type of device, [B] service focus area, [C] company size and [D] key geographical regions.
- Market Landscape: A comprehensive evaluation of combination product service providers, considering various parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] type of combination product(s), [E] type of device(s), [F] service focus area(s), [G] type of service(s) offered, [H] location of facility and [I] leading players (in terms of number of services offered).
- Company Competitiveness Analysis: A comprehensive competitive analysis of combination product service providers, examining factors, such as [A] supplier power and [B] service portfolio strength.
- Company Profiles: In-depth profiles of companies that offer services for development and testing of combination products, focusing on [A] company overviews and [B] recent developments and an informed future outlook.
- Recent Expansions: An insightful analysis of recent expansions undertaken by various combination product service providers, based on various relevant parameters, such as [A] year of expansion, [B] type of expansion, [C] location of expanded facility, [D] most active players (in terms of number of recent expansions) and [E] geographical distribution.
- Case Study: A detailed discussion on the most advanced and popular combination products, including large volume wearable injectors and prefilled syringes combination products, providing information on their developer(s) and combination product specific features.
- Likely Partner Analysis: A comprehensive analysis of more than 90 combination product developers that are likely to partner with combination product service providers. These players analyzed based on various parameters, such as [A] pipeline strength, [B] developer strength and [C] product strength.
- Go or No-Go Framework Analysis: In-depth analysis highlighting the various factors that need to be considered by combination product developers while deciding whether to develop their respective products in-house or engage the services of a service provider.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Introduction to Combination Products
- 3.3. Challenges Associated with Development of Combination Products
- 3.4. Outsourced Combination Products Operations / Services
- 3.5. Advantages of Outsourcing Operations for Combination Products
- 3.6. Key Considerations while Selecting a CDMO
- 3.7. Future Perspectives
4. COMBINATION PRODUCTS SERVICE PROVIDERS: MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Combination Products Service Providers: Overall Market Landscape
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Location of Headquarters
- 4.2.4. Analysis by Type of Combination Product
- 4.2.5. Analysis by Type of Device
- 4.2.6. Analysis by Service Focus Area(s)
- 4.2.7. Analysis by Type of Service(s) Offered
- 4.2.8. Analysis by Location of Facility
- 4.2.9. Leading Combination Products Service Providers: Analysis by Number of Service(s) Offered
- 4.2.10. 4D Bubble Representation: Analysis by Company Size, Location of Headquarters (Region), Number of Service Focus Area(s) and Number of Service(s) Offered
- 4.2.11. Mekko Representation: Analysis by Company Size and Number of Service Focus Area(s)
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Assumptions / Key Parameters
- 5.3. Methodology
- 5.4. Combination Products Service Providers: Company Competitiveness Analysis
- 5.4.1. Combination Products Service Providers based in North America
- 5.4.2. Combination Products Service Providers based in Europe and Asia
6. COMBINATION PRODUCTS SERVICE PROVIDERS: COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. EKG LABS
- 6.2.1. Company Overview
- 6.2.2. Recent Developments and Future Outlook
- 6.3. Eurofins Medical Device Testing
- 6.3.1. Company Overview
- 6.3.2. Recent Developments and Future Outlook
- 6.4. Exponent
- 6.4.1. Company Overview
- 6.4.2. Recent Developments and Future Outlook
- 6.5. Kymanox
- 6.5.1. Company Overview
- 6.5.2. Recent Developments and Future Outlook
- 6.6. Medical Engineering Technologies
- 6.6.1. Company Overview
- 6.6.2. Recent Developments and Future Outlook
- 6.7. Pace Analytical Services
- 6.7.1. Company Overview
- 6.7.2. Recent Developments and Future Outlook
- 6.8. Suttons Creek
- 6.8.1. Company Overview
- 6.8.2. Recent Developments and Future Outlook
- 6.9. West Pharmaceutical Services
- 6.9.1. Company Overview
- 6.9.2. Recent Developments and Future Outlook
- 6.10. Concluding Remarks: Initiatives Undertaken by Industry Players
7. RECENT EXPANSIONS
- 7.1. Chapter Overview
- 7.2. Combination Products Service Providers: List of Recent Expansions
- 7.2.1. Analysis by Year of Expansion
- 7.2.2. Analysis by Type of Expansion
- 7.2.3. Analysis by Location of Expanded Facility
- 7.2.4. Analysis by Type of Expansion (Country-wise)
- 7.2.5. Most Active Players: Analysis by Number of Expansions
- 7.2.6. Geographical Analysis
8. CASE STUDY: LARGE VOLUME WEARABLE INJECTORS AND PREFILLED SYRINGES COMBINATION PRODUCTS
- 8.1. Chapter Overview
- 8.2. Large Volume Wearable Injector Combination Products
- 8.2.1. Large Volume Drug Device Combination Products: Market Landscape
- 8.2.1.1. Analysis by Phase of Development
- 8.2.1.2. Analysis by Type of Device
- 8.2.1.3. Analysis by Drug Compatibility
- 8.2.1.4. Analysis by Type of Dosage
- 8.2.1.5. Analysis by Route of Administration
- 8.2.1.6. Analysis by Method of Administration
- 8.2.1.7. Analysis by Therapeutic Area
- 8.2.1.8. Analysis by Storage Volume / Capacity
- 8.2.1.9. Analysis by Usability
- 8.2.1.10. Analysis by Technology Used
- 8.2.1.11. Analysis by Mechanism of Action
- 8.2.1.12. Analysis by Type of Drug Container
- 8.2.2. Large Volume Drug Device Combination Products: Developer Landscape
- 8.2.2.1. Analysis by Year of Establishment
- 8.2.2.2. Analysis by Company Size
- 8.2.2.3. Analysis by Location of Headquarters
- 8.2.2.4. Most Active Players: Analysis by Number of Products Manufactured
- 8.2.1. Large Volume Drug Device Combination Products: Market Landscape
- 8.3. Prefilled Syringes Combination Products
- 8.3.1. Prefilled Syringe Combination Products: List of Approved Drugs
- 8.3.1.1. Analysis by Type of Drug Molecule
- 8.3.1.2. Analysis by Approval Year
- 8.3.1.3. Analysis by Geography
- 8.3.1.4. Analysis by Route of Administration
- 8.3.1.5. Analysis by Therapeutic Area
- 8.3.1.6. Analysis by Dosage Strength
- 8.3.1.7. Analysis by Other Approved Dosage Forms
- 8.3.2. Prefilled Syringe Combination Products: List of Clinical Stage Drugs
- 8.3.2.1. Analysis by Type of Drug Molecule
- 8.3.2.2. Analysis by Phase of Development
- 8.3.2.3. Analysis by Route of Administration
- 8.3.2.4. Analysis by Therapeutic Area
- 8.3.3. Prefilled Syringes Combination Products: Information on Developers
- 8.3.3.1. Analysis by Year of Establishment
- 8.3.3.2. Analysis by Company Size
- 8.3.3.3. Analysis by Location of Headquarters
- 8.3.1. Prefilled Syringe Combination Products: List of Approved Drugs
9. LIKELY PARTNER ANALYSIS
- 9.1. Chapter Overview
- 9.2. Large Volume Wearable Injectors Combination Products
- 9.2.1. Scoring Criteria and Key Assumptions
- 9.2.2. Scope and Methodology
- 9.2.3. Potential Strategic Partners
- 9.2.3.1. Most Likely Partners
- 9.2.3.2. Likely Partners
- 9.2.3.3. Less Likely Partners
- 9.3. Prefilled Syringes Combination Products
- 9.3.1. Scoring Criteria and Key Assumptions
- 9.3.2. Scope and Methodology
- 9.3.3. Potential Strategic Partners
- 9.3.3.1. Most Likely Partners
- 9.3.3.2. Likely Partners
- 9.3.3.3. Less Likely Partners
10. OUTSOURCING: GO / NO-GO FRAMEWORK
- 10.1. Chapter Overview
- 10.2. Outsourcing: Go / No-Go Framework
- 10.3. Prefilled Syringes Combination Products Developers: Go / No-Go Framework
- 10.3.1. Assumptions and Key Parameters
- 10.3.2. Methodology
- 10.3.3. Results and Interpretations
- 10.3.3.1. Outsourcing: Go / No-Go Framework for Very Small and Small Companies
- 10.3.3.2. Outsourcing: Go / No-Go Framework for Mid-sized Companies
- 10.3.3.3. Outsourcing: Go / No-Go Framework for Large Companies
11. MARKET FORECAST
- 11.1. Chapter Overview
- 11.2. Key Assumptions and Forecast Methodology
- 11.3. Global Combination Products Service Providers Market, Till 2035
- 11.3.1. Global Combination Products Service Providers Market: Distribution by Type of Device
- 11.3.1.1. Combination Products Service Providers Market for Large Volume Wearable Injectors
- 11.3.1.2. Combination Products Service Providers Market for Autoinjector Combination Products
- 11.3.1.3. Combination Products Service Providers Market for Prefilled Syringe
- 11.3.1.4. Combination Products Service Providers Market for Other Types of Combination Products
- 11.3.2. Global Combination Products Service Providers Market: Distribution by Service Focus Area
- 11.3.2.1. Combination Products Service Providers Market for Particle Testing
- 11.3.2.2. Combination Products Service Providers Market for Performance Testing
- 11.3.2.3. Combination Products Service Providers Market for Product Development
- 11.3.3. Global Combination Products Service Providers Market: Distribution by Company Size
- 11.3.3.1. Combination Products Service Providers Market for Very Small Companies
- 11.3.3.2. Combination Products Service Providers Market for Small Companies
- 11.3.3.3. Combination Products Service Providers Market for Mid-sized Companies
- 11.3.3.4. Combination Products Service Providers Market for Large Companies
- 11.3.3.5. Combination Products Service Providers Market for Very Large Companies
- 11.3.4 Combination Products Service Providers Market: Distribution by Region
- 11.3.4.1. Combination Products Service Providers Market in North America, Till 2035
- 11.3.4.2. Combination Products Service Providers Market in Europe, Till 2035
- 11.3.4.3. Combination Products Service Providers Market in Asia, Till 2035
- 11.3.4.4. Combination Products Service Providers in Rest of the World, Till 2035
- 11.3.1. Global Combination Products Service Providers Market: Distribution by Type of Device



















