
|
시장보고서
상품코드
1821508
생물제제 수탁제조 시장 : 업계 동향과 세계 예측(-2035년) - 제공 서비스 유형별, 제조 생물제제별, 사용 발현 시스템별, 사업 규모별, 기업 규모별, 주요 지역별Biologics Contract Manufacturing Market: Industry Trends and Global Forecasts, Till 2035 - Distribution by Type of Service(s) Offered, Biologic Manufactured, Expression System Used, Scale of Operation, Company Size, and Key Geographical Regions |
||||||
생물제제 수탁제조 시장 : 개요
세계 바이오의약품 위탁생산 시장 규모는 2035년까지 예측 기간 동안 8.8%의 연평균 복합 성장률(CAGR)로 확대되어 현재 238억 달러에서 2035년까지 550억 달러로 성장할 것으로 예측됩니다.
시장 세분화에서는 시장 규모 및 기회 분석을 다음과 같은 매개 변수로 구분합니다.
서비스 제공 유형별
- API 제조
- FDF 제조
제조하는 생물학적 제제별
- 항체
- 세포치료제
- 백신
- 기타
사용 발현 시스템별
- 포유류
- 미생물
- 기타
사업 규모별
- 전임상/임상
- 상업
기업 규모별
- 소규모
- 중규모
- 대기업 및 초대형 기업
지역별
- 북미
- 유럽
- 아시아태평양
- 중동 및 북아프리카
- 라틴아메리카
생물제제 수탁제조 시장 성장 및 동향
생물제제 수탁제조 분야는 특이성, 유효성, 안전성 등 생물학적 제제가 제공하는 몇 가지 장점으로 인해 최근 괄목할 만한 성장세를 보이고 있습니다. 바이오의약품 산업은 저분자 의약품에서 단일클론항체, 백신, 세포-유전자치료제, 바이오시밀러 등 복잡한 생물학적 제제로의 전환을 목격해 왔습니다는 점은 특기할 만합니다. 실제로 USFDA는 2024년 50개 이상의 생물학적 제제(단클론 항체 및 재조합 단백질 포함)를 승인했습니다. 이러한 새로운 치료제 파이프라인 증가와 생물학적 제제 승인 건수 증가와 함께 외부 제조 능력에 대한 수요가 증가하고 있습니다.
생물학적 제제의 성공에도 불구하고, 생물학적 제제의 제조는 기술적으로 어렵고, 긴 개발 기간, 규제 및 컴플라이언스 관련 문제, 최종 제품의 품질 속성과 관련된 불일치, 전문 시설, 장비 및 전문 지식에 대한 막대한 자본 투자가 필요합니다. 그 결과, 많은 바이오의약품 개발 기업들은 바이오프로세스 개발 및 최적화를 포함한 종합적인 솔루션을 찾기 위해 위탁 생산업체에 의존하고 있습니다. 이 분야에서 아웃소싱이 실용적이고 유리한 비즈니스 모델로 인식되고 있기 때문에 생물제제 수탁제조 시장은 예측 기간 동안 큰 폭의 성장이 예상됩니다.
생물제제 수탁제조 시장 주요 인사이트
본 보고서는 생물제제 수탁제조 시장의 현황을 조사하고, 업계 내 잠재적인 성장 기회를 파악합니다. 주요 조사 결과는 다음과 같습니다.
- 현재 305개 이상의 위탁생산기관(CMO)이 생물학적 제제 제조에 종사하고 있으며, 그 중 90% 이상이 FDF 제조 서비스를 제공합니다.
- 이해관계자의 약 70%는 고객의 다양한 요구에 부응하기 위해 다양한 규모의 사업을 전개하고 있습니다. 특히 포유류 세포 기반 발현 시스템은 CMO들 사이에서 인기 있는 선택으로 떠오르고 있습니다.
- 틈새 신약군을 표방하는 서비스 제공업체 간의 경쟁은 치열하며, 그 주요 요인은 과거 몇 가지 블록버스터 치료제의 성공에 기인합니다.
- 지난 10년간 생물제제 수탁제조 업계에서는 아시아태평양 개발도상국에 제조시설을 설치하는 기업이 증가하면서 트렌드가 변화하고 있습니다.
- 지난 5년간 695건 이상의 생물제제 수탁제조 계약을 체결했으며, 대부분 백신, 항체, 세포치료제 제조를 위한 계약이 대부분입니다.
- 경쟁력을 유지하고 원스톱 숍으로서의 입지를 구축하기 위해, 진출기업들은 기존 역량과 서비스 포트폴리오를 확장하고 있습니다.
- 생물제제 수탁제조과 관련된 거대한 기회를 고려하여 투자자들은 지난 8년간 90건 이상의 펀딩을 통해 75억 달러의 자금을 적극적으로 제공했습니다.
- 수요 증가에 힘입어 CMO는 주로 틈새 생물학적 제제를 대상으로 기존 역량과 능력을 확장하기 위해 세심한 투자를 해왔습니다. 이러한 추세는 미국과 중국에서 가장 두드러지게 나타나고 있습니다.
- 이러한 추세는 미국과 중국에서 가장 두드러집니다. 주요 제약사별로 시행된 이니셔티브는 215개 이상이며, 그 중 80% 이상이 제휴 및 확장에 중점을 둔 이니셔티브였습니다.
- 기존 설비 능력은 현재 생물학적 제제의 연간 수요를 충족시키기에 충분하지만, CMO는 장기적인 수요를 충족시키기 위해 설비 용량 증설에 투자할 가능성이 높을 것으로 예측됩니다.
- 전 세계 생물제제 수탁제조 능력은 다양한 지역에 분산되어 있으며, 특히 대기업이 전체의 80%를 차지하고 있습니다.
- 생물학적 제제 파이프라인 증가와 아웃소싱 지향 증가로 생물제제 수탁제조 서비스 시장은 앞으로도 안정적인 성장이 예상됩니다.
- 더 많은 개발업체들이 제조 업무의 다양한 측면을 아웃소싱함에 따라 생물제제 수탁제조 시장은 향후 10년간 연평균 8.8%의 성장률을 보일 것으로 예측됩니다.
생물제제 수탁제조 시장 주요 부문
제공 서비스 유형에 따라 시장은 API와 FDF로 구분됩니다. 현재 생물제제 수탁제조 시장의 대부분을 API가 차지하고 있다는 점은 주목할 만합니다. 이는 생물제제 원료의약품의 제조에는 막대한 설비투자가 필요하고, 설비비(개발 및 유지보수), 재료비, 인건비, 기타 많은 부대비용이 포함된다는 사실에 기인합니다. 따라서 이해관계자들은 API 제조에 있어 CMO의 전문 지식에 의존하고 있습니다.
생산되는 생물학적 제제 유형에 따라 시장은 항체, 세포치료제, 백신, 기타 생물학적 제제로 구분됩니다. 생물제제 수탁제조 시장에서 항체가 가장 큰 비중을 차지하고 있다는 점은 주목할 만합니다. 이는 전 세계적으로 100개 이상의 항체가 승인되었고, 항체 관련 임상시험이 증가하고 있기 때문입니다.
사용 발현 시스템별로 시장은 포유류, 미생물, 기타로 구분됩니다. 현재로서는 포유류 발현 시스템을 채택한 생물학적 제제 프로젝트에서 발생하는 수익에 의해 시장이 주도될 가능성이 높다는 점은 주목할 만합니다. 이는 높은 단백질 수율, 향상된 접힘 및 번역 후 변형, 개선된 배치 간 균일성으로 인해 이러한 시스템의 높은 사용률에 기인합니다.
사업 규모에 따라 시장은 전임상/임상 규모와 상업적 규모로 구분됩니다. 상업적 규모의 제조 부문이 전체 시장의 주요 견인차 역할을 할 것으로 예측됩니다. 또한, 전임상/임상 규모의 생물학적 제제 제조 시장은 상대적으로 높은 CAGR로 성장할 가능성이 높다는 점은 주목할 만합니다.
기업 규모에 따라 시장은 중소기업, 중견기업, 대기업 및 초대형 기업으로 구분됩니다. 대기업 및 초대형 기업이 상대적으로 높은 시장 점유율을 차지하고 있지만, 향후 몇 년 동안 중소기업을 위한 생물제제 수탁제조 시장이 크게 성장할 가능성이 높다는 점은 주목할 만합니다.
주요 지역별로 시장은 북미, 유럽, 아시아태평양, 중동 및 북아프리카, 라틴아메리카로 구분됩니다. 주목할 만한 점은 아시아태평양 시장이 향후 몇 년 동안 더 높은 CAGR로 성장할 것으로 예상된다는 점입니다.
생물제제 수탁제조 시장 진출기업 사례
- AGC Biologics
- Boehringer Ingelheim
- Catalent
- Cell Therapies
- Charles River Laboratories
- FUJIFILM Diosynth Biotechnologies
- KBI Biopharma
- Kemwell Biopharma
- Lonza
- Miltenyi Biotec
- Minaris Regenerative Medicine
- Samsung Biologics
- Sandoz
- Vetter Pharma
- Wuxi Biologics
1차 조사 개요
- 본 조사에서 제시된 의견과 인사이트는 여러 이해관계자와의 토론을 통해 도출된 결과입니다. 본 조사 보고서에는 다음과 같은 업계 이해관계자들과의 인터뷰 내용을 상세하게 수록했습니다.
- A사 대표이사
- B사 대표이사 겸 공동창업자
- C사 세포-유전자치료 부문 최고기술책임자
- D사 사장
- E사 세계 전략 마케팅 담당 시니어 디렉터
- F사 상업 전략 및 시장 인사이트 담당 선임 이사
- G사 영업 및 마케팅 세계 헤드 겸 시장개척(독일) 헤드
- H사 사업 개발 매니저
- I사 마케팅 및 영업 매니저
생물제제 수탁제조 시장 조사 대상
- 시장 규모와 기회 분석 : 본 보고서에서는 세계 바이오의약품 위탁생산 시장을(A) 제공 서비스 유형별,(B) 제조하는 바이오의약품별,(C) 사용 발현 시스템별,(D) 사업 규모별,(E) 기업 규모별,(F) 주요 지역별 주요 시장 부문에 대해 상세하게 분석하였습니다.
- 시장 상황:(A)설립 연도,(B)기업 규모,(C)본사 소재지,(D)제공 서비스 유형,(E)제조하는 생물학적 제제의 유형,(F)사업 규모,(G)사용 발현 시스템의 유형,(H)사용하는 바이오리액터의 유형,(I)바이오리액터의 작동 모드 등 여러 관련 매개변수를 기반으로 생물제제 수탁제조 시장에 참여하는 기업을 상세하게 평가합니다.
- 지역별 역량 분석 :(A) 북미,(B) 유럽,(C) 아시아태평양,(D) 기타 아시아태평양 등 주요 지역에 걸쳐 설립된 생물학적 제제 제조 시설에 대한 종합적인 분석.
- 기업 프로파일:(A)기업 개요,(B)재무 정보(가능한 경우),(C)서비스 포트폴리오,(D)제조 시설,(E)최근 동향 및 미래 전망 등의 매개 변수에 초점을 맞춘 북미, 유럽, 아시아태평양의 생물제제 수탁제조 시장에 종사하는 주요 서비스 제공업체의 주요 서비스 제공업체에 대한 상세한 기업 프로파일.
- 사례 연구 - 틈새 제약 분야:(A) 항체 약물 복합체(ADC),(B) 이중 특이성 항체,(C) 세포치료제,(D) 유전자치료제,(E) 바이러스 벡터 등 특정 틈새 제품에 초점을 맞추어 이 산업의 주요 원동력을 종합적으로 평가합니다.
- 사례 연구 - 자체 생산: 생물학적 제제 개발자가 각 제품을 자체 생산할 것인지, 아니면 생물학적 제제 CMO의 서비스를 이용할 것인지 결정할 때 고려해야 할 다양한 요소에 대한 자세한 검토.
- 제조 및 구매 프레임워크 주요 제약사들이 시행한 다양한 생물학적 제제 전문 제조 이니셔티브에 대한 상세한 조사로,(A) 이니셔티브 수,(B) 이니셔티브 연도,(C) 이니셔티브 목적,(D) 이니셔티브 유형,(E) 사업 규모,(F) 제조하는 생물학적 제제 유형 등 다양한 파라미터의 동향을 파악합니다. 유형 등 다양한 매개변수에 대한 동향을 파악할 수 있습니다.
- 파트너십 및 협업:(A)제휴 연도,(B)제휴 유형,(C)제조하는 생물학적 제제 유형,(D)치료 분야,(E)가장 활발하게 참여한 기업,(F)이 업계에서 이루어진 제휴 활동의 지역적 분포 등 몇 가지 관련 매개 변수를 기반으로 생물제제 수탁제조 시장의 최근 제휴를 자세히 분석합니다. 제휴를 상세하게 분석.
- 인수합병(M&A) :(A) 계약 연도,(B) 거래 유형,(C) 기업의 지역 분석,(D) 인수 유형,(E) 제조된 생물학적 제제 유형,(F) 주요 가치 동인 등 여러 관련 매개 변수를 기반으로 이 업계에서 이루어진 다양한 M&A를 상세하게 분석합니다.
- 최근 사업 확장:(A) 확장 연도,(B) 확장 목적,(C) 생산할 생물학적 제제 유형,(D) 확장 시설의 위치 등 몇 가지 관련 매개 변수에 대한 정보와 함께 해당 기간 동안 생물제제 수탁제조이 수행한 확장 이니셔티브에 대한 자세한 분석.
- 최근 동향 : 바이오의약품 위탁생산 시장의 최근 동향을 분석하여,(A) 자금 투자에 대한 정보,(B) 바이오의약품 제조 관련 기술 발전에 대한 정보를 강조합니다.
- 생산능력 분석 :(A) 제조업체의 규모,(B) 사용되는 발현 시스템 유형,(C) 지역에 따라 사용 가능한 용량의 분포에 초점을 맞추고, 생물학적 제제 제조를 위한 전체 설치 용량을 추정합니다.
- 수요 분석 :(A) 대상 환자군,(B) 투여 빈도,(C) 투여 강도 등 다양한 관련 파라미터를 기반으로 한 생물학적 제제의 연간 수요 정보 기반 추정.
- 총소유비용(Total Cost of Ownership) : 생물제제 수탁제조의 총소유비용에 대한 상세 분석.
- SWOT 분석 : 정교한 SWOT 분석을 통해 산업 발전에 영향을 미칠 관련 동향, 주요 촉진요인, 과제에 대한 고찰.
- 사례 연구 - 가상 제약사 바이오의약품 산업 전반에서 가상 비즈니스 모델의 개념과 그 역할에 대한 사례 연구.
목차
제1장 서문
제1장 생물제제 수탁제조 시장 개요
제2장 조사 방법
제3장 경제적 및 기타 프로젝트 특유의 고려사항
제4장 주요 요약
제5장 서론
- 본 장의 개요
- 생물제제 개요
- 생물제제를 위한 발현 시스템
- 생물제제 제조 공정
- 계약 제조 개요
- 생물제제 제조 업무 아웃소싱의 필요성
- 계약 제조 파트너를 선택할 때에 고려해야 할 중요 사항
- 향후 전망
제6장 시장 구도
- 본 장의 개요
- 생물제제 수탁제조업체 : 시장 구도
제7장 지역 능력 분석
- 본 장의 개요
- 주요 전제와 파라미터
- 생물제제 수탁제조 시설 개요
- 지역별 능력 분석 : 북미의 생물제제 수탁제조 시설
- 지역별 능력 분석 : 유럽의 생물제제 수탁제조 시설
- 지역별 능력 분석 : 아시아태평양의 생물제제 수탁제조 시설
- 지역별 능력 분석 : 기타 지역의 생물제제 수탁제조 시설
제8장 북미 생물제제 수탁제조
- 본 장의 개요
- 미국의 생물제제 계약 제조 : 규제 시나리오
- 북미의 대형 생물제제 CMO
- AGC Biologics
- Catalent
- FUJIFILM Diosynth Biotechnologies
- KBI Biopharma
- Charles River Laboratories
- 북미의 기타 주요 생물제제 CMO
- Cytiva
- Patheon
- Piramal Pharma Solutions
제9장 유럽의 생물제제 수탁제조
- 본 장의 개요
- 유럽의 생물제제 계약 제조 : 규제 시나리오
- EMA cGMP 규제
- 유럽의 주요 생물제제 CMO
- Boehringer Ingelheim(BioXcellence)
- Lonza
- Sandoz
- Vetter Pharma
- Miltenyi Biotec
- 유럽의 기타 주요 생물제제 CMO
- Novasep
- Olon
- Rentschler Biopharma
제10장 아시아태평양 및 세계 기타 지역의 생물제제 수탁제조
- 본 장의 개요
- 중국의 생물제제 수탁제조
- 중국의 생물제제 수탁제조 : 규제 시나리오
- 중국의 주요 생물제제 CMO
- WuXi Biologics
- 인도의 생물제제 계약 제조
- 인도의 생물제제 계약 제조 : 규제 시나리오
- 인도의 주요 생물제제 CMO
- Kemwell Biopharma
- 일본의 생물제제 수탁제조
- 일본의 생물제제 수탁제조 : 규제 시나리오
- 일본의 주요 생물제제 CMO
- Minaris Regenerative Medicine
- 한국의 생물제제 수탁제조
- 한국의 생물제제 수탁제조 : 규제 시나리오
- 한국의 주요 생물제제 CMO
- Samsung Biologics
- 호주의 생물제제 계약 제조
- 호주의 생물제제 계약 제조 : 규제 시나리오
- 호주의 대형 생물제제 CMO
- 세포치료
- 아시아태평양 및 기타 지역의 주요 생물제제 CMO
- AcuraBio(구칭 : Luina Bio)
- Celltrion
- Takara Bio
제11장 틈새 생물제제 부문
- 본 장의 개요
- 이중특이성 항체
- 항체약물접합체(ADC)
- 세포치료
- 유전자 치료
- 바이러스 벡터
- 플라스미드 DNA
제12장 사례 연구 : 바이오시밀러 아웃소싱
- 본 장의 개요
- 바이오시밀러 개요
- 바이오시밀러 개발 단계
- 바이오시밀러 라이선싱에 관한 규제 요건
- 제조 업무 아웃소싱의 필요성
- 바이오시밀러가 세계 계약 제조 시장에 미치는 영향
- 바이오시밀러 계약 제조 서비스 제공업체
- 바이오시밀러 제조업무 아웃소싱에 수반하는 과제
제13장 사례 연구 : 저분자 의약품 및 고분자 의약품/치료법 비교
- 본 장의 개요
- 저분자 및 고분자 의약품/치료법
제14장 사례 연구 : 사내 제조
제15장 제조 va. 구입 의사 의사결정 프레임워크
제16장 대형 제약회사의 이니셔티브
제17장 파트너십 및 협업
제18장 합병과 인수
제19장 최근 확장
제20장 최근 동향
제21장 능력 분석
제22장 수요 분석
제23장 생물제제 수탁제조 조직의 총소유비용
제24장 세계의 생물제제 수탁제조 시장
제25장 생물제제 수탁제조 시장(제공 서비스 유형별)
제26장 생물제제 수탁제조 시장(제조 생물제제별)
제27장 생물제제 수탁제조 시장(사용 발현 시스템별)
제28장 생물제제 수탁제조 시장(사업 규모별)
제29장 생물제제 수탁제조 시장(기업 규모별)
제30장 생물제제 수탁제조 시장(지역별)
제31장 생물제제 수탁제조 시장(주요 기업별)
제32장 사례 연구 : 가상 제약회사
제33장 SWOT 분석
제34장 생물제제 CMO 시장 전망
제35장 결론
제36장 경영진 인사이트
제37장 부록 I : 표 데이터
제38장 부록 II : 기업 및 조직 리스트
제39장 부록 III : 파트너십과 협력 상세
LSH 25.09.30Biologics Contract Manufacturing Market: Overview
As per Roots Analysis, the global biologics contract manufacturing market is estimated to grow from USD 23.8 billion in the current year to USD 55.0 billion by 2035, at a CAGR of 8.8% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Service Offered
- API Manufacturing
- FDF Manufacturing
Type of Biologic Manufactured
- Antibodies
- Cell Therapies
- Vaccines
- Other Biologics
Type of Expression System Used
- Mammalian
- Microbial
- Others
Scale of Operation
- Preclinical / Clincal
- Commercial
Company Size
- Small
- Mid-sized
- Large and Very Large
Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and North Africa
- Latin America
Biologics Contract Manufacturing Market: Growth and Trends
The biologics contract manufacturing sector has seen significant growth in recent years due to the several benefits offered by biological products, such as specificity, efficacy and safety. It is worth mentioning that the biopharmaceutical industry has witnessed a shift from small-molecule drugs to complex biologics like monoclonal antibodies, vaccines, cell and gene therapies, and biosimilars. In fact, the USFDA approved over 50 biological products (including monoclonal antibodies and recombinant proteins) in 2024. This growing pipeline of new therapies, coupled with the increasing number of biologics approvals, drives the demand for external manufacturing capacity.
Despite the success of biopharmaceutical products, producing biologics is technically challenging and requires significant capital investment in specialized facilities, equipment, and expertise, such as long development timelines, regulatory and compliance-related issues, and inconsistencies related to the quality attributes of the final product. As a result, an increasing number of biopharmaceutical drug developers are relying on contract manufacturers for comprehensive solutions, encompassing bioprocess development and optimization. With outsourcing becoming increasingly recognized as a practical and advantageous business model in this sector, substantial market growth for biologics contract manufacturing is expected throughout the forecast period.
Biologics Contract Manufacturing Market: Key Insights
The report delves into the current state of the biologics contract manufacturing market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Currently, more than 305 contract manufacturing organizations (CMOs) are engaged in the production of biologics; over 90% of such players provide FDF manufacturing services.
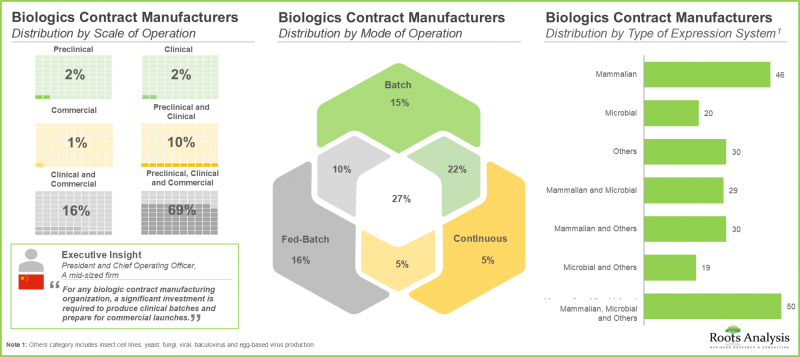
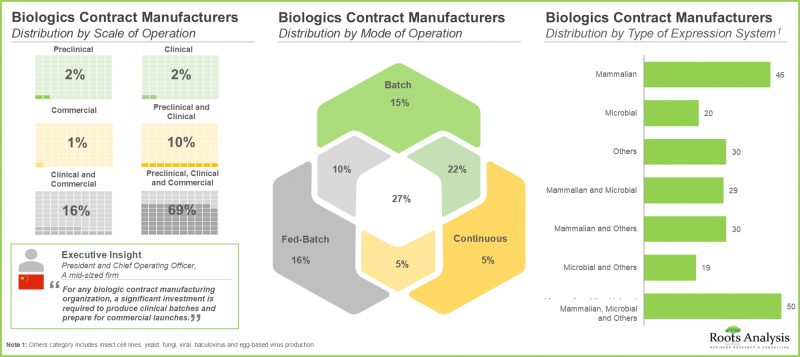
- Around 70% of the stakeholders operate at all scales of operation to cater to the diverse needs of customers; notably, mammalian cell-based expression systems have emerged as a popular choice among CMOs.
- The competition among service providers that claim to be focused on the niche and upcoming drug classes is fierce; it is primarily influenced by the success of several blockbuster therapies in the recent past.
- In the past decade, a shift in trend has been observed in the biopharmaceutical contract manufacturing industry as more players have set up their manufacturing facilities in developing regions across Asia-Pacific.
- Over the last five years, more than 695 deals have been inked by biologics CMOs; most of the collaborations were inked for the manufacturing of vaccines, antibodies and cell therapies.
- In order to maintain a competitive edge and establish themselves as one-stop-shops, players are expanding their existing capabilities and service portfolios; the domain has witnessed over 135 mergers and acquisitions.
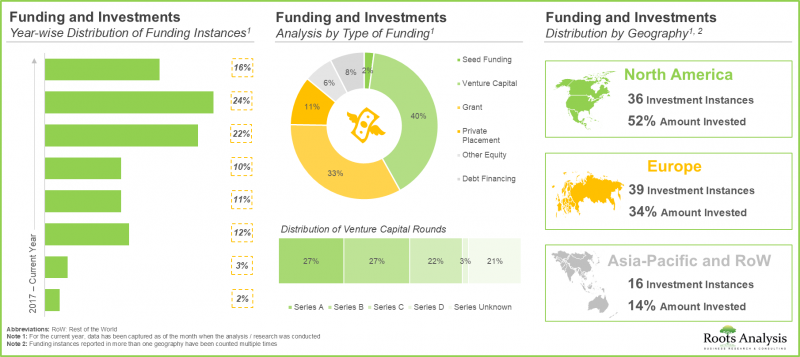
- Considering the enormous opportunities associated with biologics contract manufacturing, investors have actively extended funds, amounting to USD 7.5 billion, across more than 90 funding instances in the past eight years.
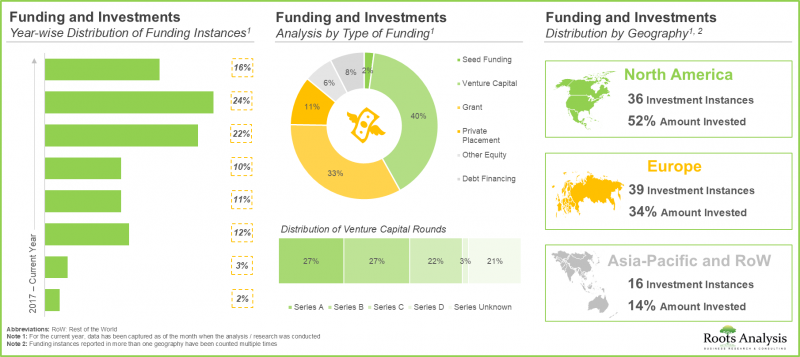
- Driven by the growing demand, CMOs have made elaborate investments to expand their existing capacities and capabilities, primarily for niche biologics; this trend is most pronounced in the US and China.
- More than 215 initiatives were undertaken by big pharma players; more than 80% of such initiatives were focused on partnerships and expansions.
- Though the existing installed capacity is sufficient to meet the current annual demand for biologics, we anticipate that CMOs are likely to invest in installing incremental capacity to meet the long-term demand.
- The global installed biopharmaceutical contract manufacturing capacity is spread across various geographies; notably, large players account for 80% of the total capacity.
- With the growing pipeline of biologics and the increased preference for outsourcing, the biopharmaceutical contract manufacturing services market is anticipated to witness steady growth in the foreseen future.
- As more developers outsource various aspects of their respective manufacturing operations, we expect the biologics CMOs market to grow at an annualized rate of 8.8% in the coming decade.
Biologics Contract Manufacturing Market: Key Segments
Contract Manufacturing Market for API is Likely to Dominate the Biologics Contract Manufacturing Market During the Forecast Period
Based on the type of service(s) offered, the market is segmented into API and FDF. It is worth highlighting that majority of the current biologics contract manufacturing market is captured by APIs. This can be attributed to the fact that manufacturing of biopharmaceuticals API demand significant capital investments, which include facility costs (development and maintenance), material costs, labor costs and a number of other ancillary expenses. Therefore, stakeholders rely on the expertise of CMOs for API production.
Cell Therapies is the Fastest Growing Segment of the Biologics Contract Manufacturing Market During the Forecast Period
Based on the type of biologic manufactured, the market is segmented into antibodies, cell therapies, vaccines and other biologics. It is worth highlighting the antibodies capture the maximum share within the biopharmaceutical contract manufacturing market. This can be attributed to the fact that more than 100 antibodies have been approved across the globe and an increasing number of clinical trials related to antibodies are also underway.
Mammalian Expression System is Expected to Capture the Highest Share of the Biologics Contract Manufacturing Market During the Forecast Period
Based on the type of expression system used, the market is segmented into mammalian, microbial and others. It is worth highlighting that currently, the market is likely to be driven by revenues generated through biopharmaceutical projects employing mammalian expression systems. This can be attributed to the higher usage of such systems owing to their high protein yielding ability, enhanced folding and post-translational modifications, and improved batch-to-batch uniformity.
By Scale of Operation, Commercial Scale is Likely to Dominate the Biologics Contract Manufacturing Market During the Forecast Period
Based on the scale of operation, the market is segmented into preclinical / clinical and commercial scale. The commercial scale manufacturing segment is projected to be the primary driver of the overall market. Further, it is worth highlighting that the biologics manufacturing market at preclinical / clinical scale is likely to grow at a relatively higher CAGR.
Large and Very Large Companies Hold Maximum Share within the Biologics Manufacturing Market
Based on company size, the market is segmented into small companies, mid-sized, and large and very large companies. While large and very large companies account for a relatively higher market share, it is worth highlighting that the biologics contract manufacturing market for small companies is likely to witness substantial market growth in the coming years.
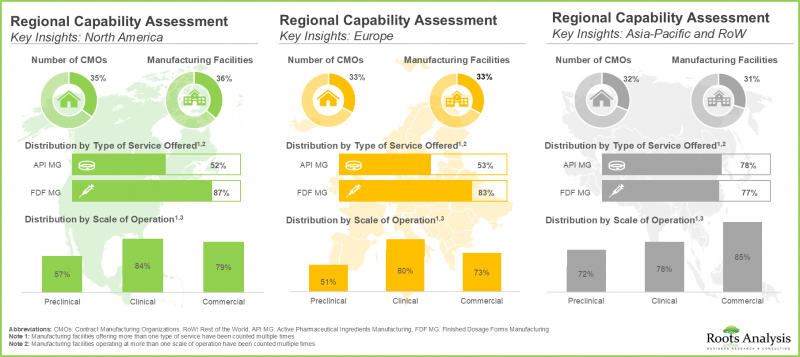
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific, Middle East and North Africa, and Latin America. It is worth highlighting that over the years, the market in Asia-Pacific is expected to grow at a higher CAGR.
Example Players in the Biologics Contract Manufacturing Market
- AGC Biologics
- Boehringer Ingelheim
- Catalent
- Cell Therapies
- Charles River Laboratories
- FUJIFILM Diosynth Biotechnologies
- KBI Biopharma
- Kemwell Biopharma
- Lonza
- Miltenyi Biotec
- Minaris Regenerative Medicine
- Samsung Biologics
- Sandoz
- Vetter Pharma
- Wuxi Biologics
Primary Research Overview
- The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:
- Chief Executive Officer, Company A
- Chief Executive Officer and Co-Founder, Company B
- Chief Technical Officer, Cell and Gene Therapy, Company C
- President, Company D
- Senior Director, Global Strategic Marketing, Company E
- Senior Director of Commercial Strategy and Market Insights, Company F
- Global Head of Sales and Marketing and Head of Business Development (Germany), Company G
- Business Development Manager, Company H
- Manager Marketing and Sales, Company I
Biologics Contract Manufacturing Market: Research Coverage
- Market Sizing and Opportunity Analysis: The report features a thorough analysis of the global biologics contract manufacturing market, in terms of the key market segments, including [A] type of service(s) offered, [B] type of biologic manufactured, [C] type of expression system used, [D] scale of operation, [E] company size and [F] key geographical regions.
- Market Landscape: An in-depth assessment of the companies involved in biologics contract manufacturing market, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] type of service offered, [E] type of biologic manufactured, [F] scale of operation, [G] type of expression systems used, [H] type of bioreactor used and [I] mode of operation of bioreactor.
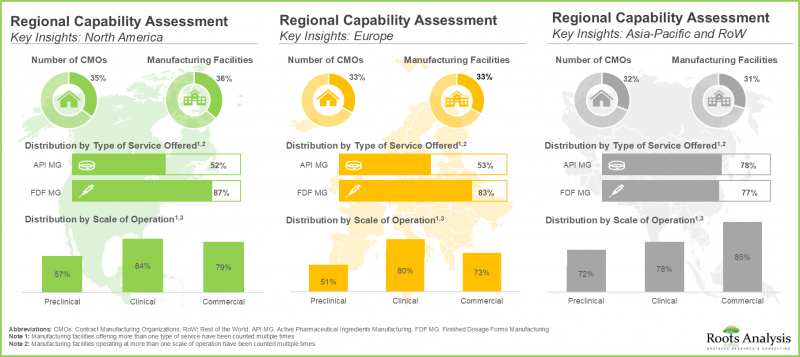
- Regional Capability Analysis: A comprehensive analysis of biopharmaceutical manufacturing facilities established across the key geographical regions, such as [A] North America [B] Europe [C] Asia-Pacific, and [D] rest of the world.
- Company Profiles: Detailed profiles of key service providers across North America, Europe and Asia-Pacific engaged in the biologics contract manufacturing market, focused on parameters such as [A] company overview, [B] financial information (if available), [C] service portfolio, [D] manufacturing facilities and [E] recent developments and an informed future outlook.
- Case Study - Niche Pharmaceutical Sectors: A comprehensive evaluation of the primary enablers within this industry, highlighting specific niche products such as [A] antibody-drug conjugates (ADCs), [B] bispecific antibodies, [C] cell therapies, [D] gene therapies, and [E] viral vectors.
- Case Study - In-house Manufacturing: A detailed review of various factors that need to be taken into consideration by biopharmaceutical developers while deciding whether to manufacture their respective products in-house or engage the services of a biologics CMO.
- Make Versus Buy Framework: An elaborate study of the various biopharmaceutical-focused manufacturing initiatives undertaken by top big pharma players, highlighting trends across various parameters, such as [A] number of initiatives, [B] year of initiative, [C] purpose of initiative, [D] type of initiative, [E] scale of operation and [F] type of biologic manufactured.
- Partnerships and Collaborations: An in-depth analysis of the recent collaborations within the biologics contract manufacturing market, based on several relevant parameters, such as [A] year of partnership, [B] type of partnership, [C] type of biologic manufactured, [D] therapeutic area, [E] most active players and [F] regional distribution of partnership activity that have taken place in this industry.
- Mergers and Acquisitions: A detailed analysis of the various mergers and acquisitions that have taken place within this industry, based on several relevant parameters, [A] such as year of agreement, [B] type of deal, [C] geographical location of companies, [D] type of acquisition, [E] type of biologic manufactured and [F] key value drivers.
- Recent Expansions: A detailed analysis of expansion initiatives undertaken by biologics CMO, during the period along with information on several relevant parameters, such as [A] year of expansion, [B] purpose of expansion, [C] type of biologic manufactured and [D] location of expanded facility.
- Recent Developments: An analysis of the recent developments within the biologics contract manufacturing market, highlighting information on the [A] funding investments made and [B] information on the technology advancements related to biomanufacturing.
- Capacity Analysis: An estimate of the overall installed capacity for the manufacturing of biopharmaceuticals, highlighting the distribution of the available capacity, based on [A] size of manufacturer, [B]type of expression system used and [C] geography.
- Demand Analysis: An informed estimate of the annual demand for biologics, based on various relevant parameters, such as [A] target patient population, [B] dosing frequency and [C] dose strength.
- Total Cost of Ownership: A detailed analysis of the total cost of ownership for biologics CMO.
- SWOT Analysis: A discussion on affiliated trends, key drivers and challenges, under an elaborate SWOT analysis, which are likely to impact the industry's evolution.
- Case Study - Virtual Pharmaceutical Companies: A case study on the virtual business model concept, along with its role in the overall biopharmaceutical industry.
Key Questions Answered in this Report
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
Reasons to Buy this Report
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
Additional Benefits
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
1.1. Biopharmaceutical Contract Manufacturing Market Overview
- 1.2. Key Market Insights
- 1.3. Scope of the Report
- 1.4. Research Methodology
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.3. Project Methodology
- 2.4. Forecast Methodology
- 2.5. Robust Quality Control
- 2.6. Key Market Segmentations
- 2.7. Key Considerations
- 2.7.1. Demographics
- 2.7.2. Economic Factors
- 2.7.3. Government Regulations
- 2.7.4. Supply Chain
- 2.7.5. COVID Impact / Related Factors
- 2.7.6. Market Access
- 2.7.7. Healthcare Policies
- 2.7.8. Industry Consolidation
3. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Forecasted Estimates
- 3.2.2. Currency Coverage
- 3.2.2.1. Overview of Major Currencies Affecting the Market
- 3.2.2.2. Impact of Currency Fluctuations on the Industry
- 3.2.3. Foreign Exchange Impact
- 3.2.3.1. Evaluation of Foreign Exchange Rates and Their Impact on Market
- 3.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. EXECUTIVE SUMMARY
5. INTRODUCTION
- 5.1. Chapter Overview
- 5.2. Overview of Biopharmaceuticals
- 5.3. Expression Systems for Biopharmaceuticals
- 5.3.1. Insect Expression Systems
- 5.3.2. Mammalian Expression Systems
- 5.3.3. Microbial Expression Systems
- 5.3.3.1. Bacterial Expression Systems
- 5.3.3.2. Fungal Expression Systems
- 5.3.3.3. Yeast Expression Systems
- 5.3.4. Plant Expression Systems
- 5.3.5. Mammalian versus Microbial Expression Systems
- 5.4. Manufacturing Process of Biopharmaceuticals
- 5.4.1. Upstream Processing
- 5.4.2. Fermentation
- 5.4.3. Downstream Processing
- 5.5. Overview of Contract Manufacturing
- 5.6. Need for Outsourcing Biopharmaceutical Manufacturing Operations
- 5.6.1. Commonly Outsourced Manufacturing Operations for Biopharmaceuticals
- 5.6.2. Advantages of Outsourcing Biopharmaceutical Manufacturing Operations
- 5.6.3. Risks and Challenges Associated with Outsourcing Biopharmaceutical Manufacturing Operations
- 5.7. Key Considerations While Selecting a Contract Manufacturing Partner
- 5.8. Future Perspectives
6. MARKET LANDSCAPE
- 6.1. Chapter Overview
- 6.2. Biopharmaceutical Contract Manufacturers: Overall Market Landscape
- 6.2.1. Analysis by Year of Establishment
- 6.2.2. Analysis by Company Size
- 6.2.3. Analysis by Location of Headquarters
- 6.2.4. Analysis by Type of Service Offered
- 6.2.5. Analysis by Type of Biologic Manufactured
- 6.2.6. Analysis by Scale of Operation
- 6.2.7. Analysis by Type of Expression System Used
- 6.2.8. Analysis by Type of Bioreactor Used
- 6.2.9. Analysis by Mode of Operation of Bioreactor
7. REGIONAL CAPABILITY ANALYSIS
- 7.1. Chapter Overview
- 7.2. Key Assumptions and Parameter
- 7.3. Overview of Biopharmaceutical Contract Manufacturing Facilities
- 7.3.1. Analysis by Type of Service Offered
- 7.3.2. Analysis by Scale of Operation
- 7.4. Regional Capability Analysis: Biopharmaceutical Contract Manufacturing Facilities in North America
- 7.5. Regional Capability Analysis: Biopharmaceutical Contract Manufacturing Facilities in Europe
- 7.6. Regional Capability Analysis: Biopharmaceutical Contract Manufacturing Facilities in Asia-Pacific
- 7.7. Regional Capability Analysis: Biopharmaceutical Contract Manufacturing Facilities in Rest of the World
8. BIOPHARMACEUTICAL CONTRACT MANUFACTURING IN NORTH AMERICA
- 8.1. Chapter Overview
- 8.2. Biopharmaceutical Contract Manufacturing in the US: Regulatory Scenario
- 8.3. Leading Biopharmaceutical CMOs in North America
- 8.3.1. AGC Biologics
- 8.3.1.1. Company Overview
- 8.3.1.2. Service Portfolio
- 8.3.1.2.1. Process Development
- 8.3.1.2.2. cGMP Manufacturing
- 8.3.1.2.3. Quality and Regulatory Services
- 8.3.1.2.4. Process Validation
- 8.3.1.3. Financial Information
- 8.3.1.4. Manufacturing Facilities
- 8.3.1.5. Recent Developments and Future Outlook
- 8.3.2. Catalent
- 8.3.2.1. Company Overview
- 8.3.2.2. Service Portfolio
- 8.3.2.2.1. Cell Line Development
- 8.3.2.2.2. Biomanufacturing
- 8.3.2.2.3. ADCs and Bioconjugates Manufacturing
- 8.3.2.2.4. Biosimilars Development and Manufacturing
- 8.3.2.2.5. Fill / Finish Solutions and Delivery Services
- 8.3.2.2.6. Analytical Services
- 8.3.2.3. Clinical Supply Services
- 8.3.2.4. Financial Information
- 8.3.2.5. Manufacturing Facilities
- 8.3.2.6. Recent Developments and Future Outlook
- 8.3.3. FUJIFILM Diosynth Biotechnologies
- 8.3.3.1. Company Overview
- 8.3.3.2. Service Portfolio
- 8.3.3.2.1. Strain Development
- 8.3.3.2.2. Process Development
- 8.3.3.2.3. cGMP Manufacturing
- 8.3.3.2.4. Analytical Solutions
- 8.3.3.3. Financial Information
- 8.3.3.4. Manufacturing Facilities
- 8.3.3.5. Recent Developments and Future Outlook
- 8.3.4. KBI Biopharma
- 8.3.4.1. Company Overview
- 8.3.4.2. Service Portfolio
- 8.3.4.2.1. Process Development
- 8.3.4.2.2. Analytical Development
- 8.3.4.2.3. GMP Manufacturing
- 8.3.4.2.4. Clinical Cell Therapy Support
- 8.3.4.3. Manufacturing Facilities
- 8.3.4.4. Recent Developments and Future Outlook
- 8.3.5. Charles River Laboratories
- 8.3.5.1. Company Overview
- 8.3.5.2. Service Portfolio
- 8.3.5.2.1. Cell Sourcing
- 8.3.5.2.2. Cell and Gene Therapy Solutions
- 8.3.5.2.3. Biologics Testing Solutions
- 8.3.5.2.4. Avian Vaccine Services
- 8.3.5.2.5. QC Microbial Solutions
- 8.3.5.2.6. Scientific and Regulatory Advisory Services
- 8.3.5.3. Financial Information
- 8.3.5.4. Manufacturing Facilities
- 8.3.5.5. Recent Developments and Future Outlook
- 8.3.1. AGC Biologics
- 8.4. Other Leading Biopharmaceutical CMOs in North America
- 8.4.1. Cytiva
- 8.4.1.1. Company Overview
- 8.4.2. Patheon
- 8.4.2.1. Company Overview
- 8.4.3. Piramal Pharma Solutions
- 8.4.3.1. Company Overview
- 8.4.1. Cytiva
9. BIOPHARMACEUTICAL CONTRACT MANUFACTURING IN EUROPE
- 9.1. Chapter Overview
- 9.2. Biopharmaceutical Contract Manufacturing in Europe: Regulatory Scenario
- 9.2.1. EMA's cGMP Regulations
- 9.3. Leading Biopharmaceutical CMOs in Europe
- 9.3.1. Boehringer Ingelheim (BioXcellence)
- 9.3.1.1. Company Overview
- 9.3.1.2. Service Portfolio
- 9.3.1.2.1. Process Development
- 9.3.1.2.1.1. Expression Systems
- 9.3.1.2.1.2. Upstream Technology
- 9.3.1.2.1.3. Downstream Technology
- 9.3.1.2.1.4. Other Process Development Services
- 9.3.1.2.2. Quality Assurance
- 9.3.1.2.3. Fill / Finish Services
- 9.3.1.2.1. Process Development
- 9.3.1.3. Financial Information
- 9.3.1.4. Manufacturing Facilities
- 9.3.1.5. Recent Developments and Future Outlook
- 9.3.2. Lonza
- 9.3.2.1. Company Overview
- 9.3.2.2. Service Portfolio
- 9.3.2.3. Manufacturing Services
- 9.3.2.4. Financial Information
- 9.3.2.5. Manufacturing Facilities
- 9.3.2.6. Recent Developments and Future Outlook
- 9.3.3. Sandoz
- 9.3.3.1. Company Overview
- 9.3.3.2. Service Portfolio
- 9.3.3.3. Financial Information
- 9.3.3.4. Manufacturing Facilities
- 9.3.3.5. Recent Developments and Future Outlook
- 9.3.4. Vetter Pharma
- 9.3.4.1. Company Overview
- 9.3.4.2. Service Portfolio
- 9.3.4.3. Manufacturing Facilities
- 9.3.4.4. Recent Developments and Future Outlook
- 9.3.5. Miltenyi Biotec
- 9.3.5.1. Company Overview
- 9.3.5.2. Service Portfolio
- 9.3.5.3. Manufacturing Facilities
- 9.3.5.4. Recent Developments and Future Outlook
- 9.3.1. Boehringer Ingelheim (BioXcellence)
- 9.4. Other Leading Biopharmaceutical CMOs in Europe
- 9.4.1. Novasep
- 9.4.1.1. Company Overview
- 9.4.2. Olon
- 9.4.2.1. Company Overview
- 9.4.3. Rentschler Biopharma
- 9.4.3.1. Company Overview
- 9.4.1. Novasep
10. BIOPHARMACEUTICAL CONTRACT MANUFACTURING IN ASIA-PACIFIC AND REST OF THE WORLD
- 10.1. Chapter Overview
- 10.2. Biopharmaceutical Contract Manufacturing in China
- 10.2.1. Biopharmaceutical Contract Manufacturing in China: Regulatory Scenario
- 10.3. Leading Biopharmaceutical CMOs in China
- 10.3.1. WuXi Biologics
- 10.3.1.1. Company Overview
- 10.3.1.2. Service Portfolio
- 10.3.1.2.1. Discovery Services
- 10.3.1.2.2. Development Services
- 10.3.1.2.3. Testing Services
- 10.3.1.2.4. Clinical Manufacturing Services
- 10.3.1.3. Financial Information
- 10.3.1.4. Manufacturing Facilities
- 10.3.1.5. Recent Developments and Future Outlook
- 10.3.1. WuXi Biologics
- 10.4. Biopharmaceutical Contract Manufacturing in India
- 10.4.1. Biopharmaceutical Contract Manufacturing in India: Regulatory Scenario
- 10.5. Leading Biopharmaceutical CMOs in India
- 10.5.1. Kemwell Biopharma
- 10.5.1.1. Company Overview
- 10.5.1.2. Service Portfolio
- 10.5.1.2.1. Development Services for Biopharmaceuticals
- 10.5.1.2.2. Manufacturing Services for Biopharmaceuticals
- 10.5.1.3. Manufacturing Facilities
- 10.5.1.4. Recent Developments and Future Outlook
- 10.5.1. Kemwell Biopharma
- 10.6. Biopharmaceutical Contract Manufacturing in Japan
- 10.6.1. Biopharmaceutical Contract Manufacturing in Japan: Regulatory Scenario
- 10.7. Leading Biopharmaceutical CMOs in Japan
- 10.7.1. Minaris Regenerative Medicine
- 10.7.1.1. Company Overview
- 10.7.1.2. Service Portfolio
- 10.7.1.2.1. Manufacturing Development Services
- 10.7.1.2.2. GMP Manufacturing
- 10.7.1.3. Manufacturing Facilities
- 10.7.1.4. Recent Developments and Future Outlook
- 10.7.1. Minaris Regenerative Medicine
- 10.8. Biopharmaceutical Contract Manufacturing in South Korea
- 10.8.1. Biopharmaceutical Contract Manufacturing in South Korea: Regulatory Scenario
- 10.9. Leading Biopharmaceutical CMOs in South Korea
- 10.9.1. Samsung Biologics
- 10.9.1.1. Company Overview
- 10.9.1.2. Service Portfolio
- 10.9.1.2.1. Process Development
- 10.9.1.2.2. Analytical Services
- 10.9.1.2.3. cGMP Manufacturing Services
- 10.9.1.2.4. Aseptic Fill / Finish Services
- 10.9.1.2.5. Quality Services
- 10.9.1.3. Financial Information
- 10.9.1.4. Manufacturing Facilities
- 10.9.1.5. Recent Developments and Future Outlook
- 10.9.1. Samsung Biologics
- 10.10. Biopharmaceutical Contract Manufacturing in Australia
- 10.10.1. Biopharmaceutical Contract Manufacturing in Australia: Regulatory Scenario
- 10.11. Leading Biopharmaceutical CMOs in Australia
- 10.11.1. Cell Therapies
- 10.11.1.1. Company Overview
- 10.11.1.2. Service Portfolio
- 10.11.1.3. Manufacturing Facilities
- 10.11.1.4. Recent Developments and Future Outlook
- 10.11.1. Cell Therapies
- 10.12. Other Leading Biopharmaceutical CMOs in Asia-Pacific and Rest of the World
- 10.12.1. AcuraBio (Formerly Known as Luina Bio)
- 10.12.1.1. Company Overview
- 10.12.2. Celltrion
- 10.12.2.1. Company Overview
- 10.12.3. Takara Bio
- 10.12.3.1. Company Overview
- 10.12.1. AcuraBio (Formerly Known as Luina Bio)
11. NICHE BIOPHARMACEUTICAL SECTORS
- 11.1. Chapter Overview
- 11.2. Bispecific Antibodies
- 11.2.1. Approved and Clinical Bispecific Antibody Therapeutics: Overall Market Landscape
- 11.2.2. Bispecific Antibodies: Pipeline Analysis
- 11.2.2.1. Analysis by Phase of Development
- 11.2.2.2. Analysis by Target Indication
- 11.2.3. Bispecific Antibody Therapeutics: Technology Platforms
- 11.2.4. Key Considerations for Manufacturing and Associated Challenges
- 11.2.5. Role of CMOs in Offering Services for Bispecific Antibodies
- 11.2.5.1. CMOs Offering Services for Bispecific Antibodies
- 11.3. Antibody Drug Conjugates (ADCs)
- 11.3.1. Components of ADCs
- 11.3.1.1. Antibody
- 11.3.1.2. Cytotoxin
- 11.3.1.3. Linker
- 11.3.2. Antibody Drug Conjugates (ADCs): Pipeline Analysis
- 11.3.2.1. Analysis by Status of Development
- 11.3.2.2. Analysis by Target Disease Indication
- 11.3.2.3. Most Active Players: Analysis by Number of Therapies
- 11.3.3. Antibody Drug Conjugate Developers
- 11.3.4. Manufacturing Process
- 11.3.1. Components of ADCs
- 11.4. Cell Therapies
- 11.4.1. Cell Therapies: Overall Market Landscape
- 11.4.2. Overview of Cell Therapy Manufacturing
- 11.4.2.1. Cell Therapy Manufacturing Models
- 11.4.2.1.1. Centralized Manufacturing
- 11.4.2.1.2. Decentralized Manufacturing
- 11.4.2.1. Cell Therapy Manufacturing Models
- 11.4.3. Key Challenges for Manufacturing Cell Therapies
- 11.4.4. Key Factors Impacting Cell Therapy Manufacturing
- 11.4.4.1. Characterization
- 11.4.4.2. Cost of Goods
- 11.4.4.3. Automation of Cell Therapy Manufacturing
- 11.4.5. Cell Therapies: Pipeline Analysis
- 11.4.5.1. Analysis by Type of Cell Manufactured
- 11.4.6. Stem Cell Therapies: Analysis by Phase of Development
- 11.4.7. T-Cell Therapies: Analysis by Phase of Development
- 11.4.8. Role of CMOs in Offering Services for Cell Therapies
- 11.4.8.1. CMOs Offering Services for Cell Therapies
- 11.5. Gene Therapies
- 11.5.1. Gene Therapies: Pipeline Analysis
- 11.5.1.1. Analysis by Stage of Development
- 11.5.1.2. Analysis by Phase of Development
- 11.5.1.3. Analysis by Type of Vector Used
- 11.5.1.3.1. Clinical Pipeline
- 11.5.1.3.2. Preclinical Pipeline
- 11.5.1.4. Analysis by Therapeutic Area
- 11.5.1.4.1. Clinical and Commercial Pipeline
- 11.5.1.4.2. Preclinical Pipeline
- 11.5.2. Role of CMOs in Offering Services for Gene Therapies
- 11.5.2.1. CMOs Offering Services for Gene Therapies
- 11.5.1. Gene Therapies: Pipeline Analysis
- 11.6. Viral Vectors
- 11.6.1. Viral Vectors: Pipeline Analysis
- 11.6.1.1. Analysis by Location of Viral Vectors Manufacturing Facilities
- 11.6.1.2. Analysis by Type of Viral Vector Manufactured
- 11.6.2. Role of CMOs in Offering Services for Viral Vectors
- 11.6.2.1. CMOs Offering Services for Viral Vectors
- 11.6.1. Viral Vectors: Pipeline Analysis
- 11.7. Plasmid DNA
- 11.7.1. Plasmid DNA: Pipeline Analysis
- 11.7.1.1. Analysis by Location of Manufacturing Facilities
- 11.7.2. Role of CMOs in Offering Services for Plasmid DNA
- 11.7.2.1. CMOs Offering Services for Plasmid DNA
- 11.7.1. Plasmid DNA: Pipeline Analysis
12. CASE STUDY: OUTSOURCING OF BIOSIMILARS
- 12.1. Chapter Overview
- 12.2. Overview of Biosimilars
- 12.3. Development Stages of Biosimilars
- 12.4. Regulatory Requirements for Licensing of Biosimilars
- 12.5. Need for Outsourcing Manufacturing Operations
- 12.6. Impact of Biosimilars on the Global Contract Manufacturing Market
- 12.6.1. Biosimilars: Historical Trend of FDA Approvals
- 12.7. Biosimilars Contract Manufacturing Service Providers
- 12.8. Challenges Associated with Outsourcing of Biosimilar Manufacturing Operations
13. CASE STUDY: COMPARISON OF SMALL AND LARGE MOLECULE DRUGS / THERAPIES
- 13.1. Chapter Overview
- 13.2. Small Molecule and Large Molecule Drugs / Therapies
- 13.2.1. Comparison of General Characteristics
- 13.2.2. Comparison of Key Specifications
- 13.2.3. Comparison of Manufacturing Process
- 13.2.4. Comparison of Key Manufacturing Challenges
14. CASE STUDY: IN-HOUSE MANUFACTURING
- 14.1. Chapter Overview
- 14.2. In-House Manufacturing
- 14.2.1. Benefits Associated with In-House Manufacturing
- 14.2.2. Risks Associated with In-House Manufacturing
- 14.3. Outsourcing Trends in the Biopharmaceutical Industry
- 14.3.1. Types of Outsourcing Partners
- 14.4. Manufacturing Approaches Used for Approved Biologics
- 14.5. Choosing the Right Strategy: In-House Manufacturing versus Outsourcing
15. MAKE VERSUS BUY DECISION MAKING FRAMEWORK
- 15.1. Chapter Overview
- 15.2. Key Assumptions and Parameters
- 15.3. Biopharmaceutical Contract Manufacturers: Make versus Buy Decision Making
- 15.3.1. Scenario 1
- 15.3.2. Scenario 2
- 15.3.3. Scenario 3
- 15.3.4. Scenario 4
- 15.4. Conclusion
16. BIG PHARMA INITIATIVES
- 16.1. Chapter Overview
- 16.2. Biopharmaceutical Related Initiatives by Big Pharmaceutical Players
- 16.2.1. Analysis by Number of Initiatives
- 16.2.2. Analysis by Year of Initiative
- 16.2.3. Analysis by Purpose of Initiative
- 16.2.4. Analysis by Type of Initiative
- 16.2.4.1. Analysis by Type of Partnership
- 16.2.4.2. Analysis by Type of Expansion
- 16.2.5. Analysis by Scale of Operation
- 16.2.6. Analysis by Type of Biologic Manufactured
- 16.2.7. Analysis of Big Pharma Players by Year of Initiative
- 16.2.8. Analysis of Big Pharma Players by Purpose of Initiative
- 16.2.9. Analysis by Year and Type of Initiative
- 16.2.10. Analysis of Big Pharma Players by Region of Expansion
- 16.2.11. Analysis of Big Pharma Players by Type of Biologic Manufactured
17. PARTNERSHIPS AND COLLABORATIONS
- 17.1. Chapter Overview
- 17.2. Partnership Models
- 17.3. Biopharmaceutical Contract Manufacturing: Partnerships and Collaborations
- 17.3.1. Analysis by Year of Partnership
- 17.3.2. Analysis by Type of Partnership
- 17.3.3. Analysis by Year and Type of Partnership
- 17.3.4. Analysis by Type of Biologic Manufactured
- 17.3.5. Analysis by Year of Partnership and Type of Biologic Manufactured
- 17.3.6. Analysis by Type of Partnership and Type of Biologic Manufactured
- 17.3.7. Analysis by Scale of Operation
- 17.3.8. Analysis by Therapeutic Area
- 17.3.9. Most Active Players: Analysis by Number of Partnerships
- 17.3.10. Analysis by Geography
- 17.3.10.1. Local and International Agreements
- 17.3.10.2. Intracontinental and Intercontinental Agreements
18. MERGERS AND ACQUISITIONS
- 18.1. Chapter Overview
- 18.2. Merger and Acquisition Models
- 18.3. Biopharmaceutical Contract Manufacturing: Mergers and Acquisitions
- 18.3.1. Cumulative Year-wise Trend of Mergers and Acquisitions
- 18.3.2. Analysis by Type of Acquisition
- 18.3.3. Analysis by Geography
- 18.3.3.1. Local and International Mergers and Acquisitions
- 18.3.3.2. Intracontinental and Intercontinental Mergers and Acquisitions
- 18.3.3.3. Year-wise Trend in North America, Europe and Asia-Pacific
- 18.3.4. Most Active Acquirers: Analysis by Number of Acquisitions
- 18.3.5. Analysis by Key Value Drivers
- 18.3.6. Analysis by Year of Acquisition and Key Value Drivers
- 18.3.7. Analysis by Type of Biologic Manufactured
- 18.3.8. Analysis by Key Value Drivers and Type of Biologic Manufactured
- 18.4. Key Acquisitions: Deal Multiples
- 18.4.1. Year-wise Trend of Deal Multiple Amount
19. RECENT EXPANSIONS
- 19.1. Chapter Overview
- 19.2. Biopharmaceutical Contract Manufacturing: Recent Expansions
- 19.2.1. Analysis by Year of Expansion
- 19.2.2. Analysis by Purpose of Expansion
- 19.2.3. Analysis by Year and Purpose of Expansion
- 19.2.4. Analysis by Type of Biologic Manufactured
- 19.2.5. Analysis by Purpose of Expansion and Type of Biologic Manufactured
- 19.2.6. Analysis by Location of Expanded Facility
- 19.2.7. Most Active Players: Analysis by Number of Recent Expansions
- 19.2.8. Analysis by Purpose of Expansion and Location of Expanded Facility
- 19.2.9. Analysis by Amount Invested
- 19.2.10. Recent Expansions: Scenarios
20. RECENT DEVELOPMENTS
- 20.1. Chapter Overview
- 20.2. Types of Funding
- 20.3. Biopharmaceutical Contract Manufacturing: Funding and Investment Analysis
- 20.3.1. Analysis by Year of Funding
- 20.3.2. Analysis by Amount Invested
- 20.3.3. Analysis by Type of Funding
- 20.3.4. Analysis by Year and Type of Funding
- 20.3.5. Analysis of Funding Instances and Amount Invested by Geography (Continent)
- 20.3.6. Analysis of Funding Instances and Amount Invested by Geography (Country)
- 20.3.7. Most Active Players: Analysis by Number of Funding Instances
- 20.3.8. Most Active Players: Analysis by Total Amount Raised
- 20.3.9. Leading Investors: Analysis by Number of Funding Instances
- 20.3.10. Leading Investors: Analysis by Total Amount Raised
- 20.4. Technological Advancements
- 20.4.1. Single-Use Technology
- 20.4.2. Process Analytical Technology (PAT)
- 20.4.3. Continuous Processing
- 20.4.4. Quality by Design (QbD) in Bio-processing
- 20.4.5. Modular / Podular Biopharma Facilities
21. CAPACITY ANALYSIS
- 21.1. Chapter Overview
- 21.2. Key Assumptions and Methodology
- 21.3. Biopharmaceutical Contract Manufacturing: Global Installed Capacity
- 21.3.1. Analysis by Company Size
- 21.3.2. Analysis by Type of Expression System Used
- 21.3.3. Analysis by Geography
- 21.3.3.1. Analysis of Biopharmaceutical Contract Manufacturing Capacity in North America
- 21.3.3.2. Analysis of Biopharmaceutical Contract Manufacturing Capacity in Europe
- 21.3.3.3. Analysis of Biopharmaceutical Contract Manufacturing Capacity in Asia-Pacific
- 21.3.3.4. Analysis of Biopharmaceutical Contract Manufacturing Capacity in Rest of the World
- 21.4. Concluding Remarks
22. DEMAND ANALYSIS
- 22.1. Chapter Overview
- 22.2. Key Assumptions and Methodology
- 22.3. Global Demand for Biopharmaceuticals
- 22.4. Global Demand for Emerging Novel Biologics
- 22.4.1. Global Demand for ADC Therapeutics
- 22.4.2. Global Demand for Cell Therapy Manufacturing
23. TOTAL COST OF OWNERSHIP FOR BIOPHARMACEUTICAL CONTRACT MANUFACTURING ORGANIZATIONS
- 23.1. Chapter Overview
- 23.2. Key Parameters
- 23.3. Assumptions and Methodology
- 23.4. Total Cost of Ownership (Sample Dataset)
- 23.5. Total Cost of Ownership for Mid-sized Biopharmaceutical Contract Manufacturing Organizations, Y0-Y20
- 23.5.1. Total Cost of Ownership for Mid-sized Biopharmaceutical Contract Manufacturing Organizations: Analysis by CAPEX, Y0
- 23.5.2. Total Cost of Ownership for Mid-sized Biopharmaceutical Contract Manufacturing Organizations: Analysis by OPEX, Y1-Y20
- 23.6. Total Cost of Ownership for Large / Very Large Biopharmaceutical Contract Manufacturing Organizations, Y0-Y20
- 23.6.1. Total Cost of Ownership for Large / Very Large Biopharmaceutical Contract Manufacturing Organizations: Analysis by CAPEX, Y0
- 23.6.2. Total Cost of Ownership for Large / Very Large Biopharmaceutical Contract Manufacturing Organizations: Analysis by OPEX, Y1-Y20
24. GLOBAL BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET
- 24.1. Chapter Overview
- 24.2. Assumptions and Methodology
- 24.3. Global Biopharmaceutical Contract Manufacturing Market, Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 24.3.1. Scenario Analysis
- 24.3.1.1. Conservative Scenario
- 24.3.1.2. Optimistic Scenario
- 24.3.1. Scenario Analysis
- 24.4. Key Market Segmentations
25. BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY TYPE OF SERVICE OFFERED
- 25.1. Chapter Overview
- 25.2. Key Assumptions and Methodology
- 25.3. Biopharmaceutical Contract Manufacturing Market: Distribution by Type of Service Offered, 2018, Current Year and 2035
- 25.3.1. API Manufacturing: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 25.3.2. FDF Manufacturing: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 25.4. Data Triangulation and Validation
26. BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY TYPE OF BIOLOGIC MANUFACTURED
- 26.1. Chapter Overview
- 26.2. Key Assumptions and Methodology
- 26.3. Biopharmaceutical Contract Manufacturing Market: Distribution by Type of Biologic Manufactured, 2018, Current Year and 2035
- 26.3.1. Antibodies: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 26.3.2. Cell Therapies: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 26.3.3. Vaccines: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 26.3.4. Other Biologics: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 26.4. Data Triangulation and Validation
27. BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY TYPE OF EXPRESSION SYSTEM USED
- 27.1. Chapter Overview
- 27.2. Key Assumptions and Methodology
- 27.3. Biopharmaceutical Contract Manufacturing Market: Distribution by Type of Expression System Used, 2018, Current Year and 2035
- 27.3.1. Mammalian Expression Systems: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 27.3.2. Microbial Expression Systems: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 27.3.3. Other Expression Systems: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 27.4. Data Triangulation and Validation
28. BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SCALE OF OPERATION
- 28.1. Chapter Overview
- 28.2. Key Assumptions and Methodology
- 28.3. Biopharmaceutical Contract Manufacturing Market: Distribution by Scale of Operation, 2018, Current Year and 2035
- 28.3.1. Preclinical / Clinical Operations: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 28.3.2. Commercial Operations: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 28.4. Data Triangulation and Validation
29. BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY COMPANY SIZE
- 29.1. Chapter Overview
- 29.2. Key Assumptions and Methodology
- 29.3. Biopharmaceutical Contract Manufacturing Market: Distribution by Company Size, 2018, Current Year and 2035
- 29.3.1. Small Companies: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 29.3.2. Mid-sized Companies: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 29.3.3. Large and Very Large Companies: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 29.4. Data Triangulation and Validation
30. BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY GEOGRAPHY
- 30.1. Chapter Overview
- 30.2. Key Assumptions and Methodology
- 30.3. Biopharmaceutical Contract Manufacturing Market: Distribution by Geography, 2018, Current Year and 2035
- 30.3.1. North America: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.1.1. US: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.1.2. Canada: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.2. Europe: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.2.1. Italy: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.2.2. Germany: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.2.3. France: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.2.4. Spain: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.2.5. UK: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.2.6. Rest of Europe: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.3. Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.3.1. China: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.3.2. India: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.3.3. South Korea: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.3.4. Japan: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.3.5. Rest of Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.4. Latin America: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.5. Middle East and North Africa: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.3.1. North America: Historical Trends (Since 2018) and Forecasted Estimates (till 2035)
- 30.4. Data Triangulation and Validation
31. BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY LEADING PLAYERS
- 31.1. Chapter Overview
- 31.2. Key Assumptions and Methodology
- 31.3. Biopharmaceutical Contract Manufacturing Market: Distribution by Leading Players
- 31.4. Data Triangulation and Validation
32. CASE STUDY: VIRTUAL PHARMACEUTICAL COMPANIES
- 32.1. Chapter Overview
- 32.2. Historical Evolution of the Virtual Business Model
- 32.3. Virtual Pharmaceutical Companies as a Subset of the Overall Biopharmaceutical Industry
- 32.4. Advantages Associated with Outsourcing Operations to Virtual Service Providers
- 32.5. Key Challenges Associated with Outsourcing Operations to Virtual Service Providers
33. SWOT ANALYSIS
- 33.1. Chapter Overview
- 33.2. Strengths
- 33.3. Weaknesses
- 33.4. Opportunities
- 33.5. Threats
- 33.6. Comparison of SWOT Factors
- 33.7. Conclusion
34. FUTURE OF THE BIOPHARMACEUTICAL CMO MARKET
- 34.1. Chapter Overview
- 34.2. Outsourcing Activities to Witness Significant Growth in the Coming Years
- 34.3. Shift from One-time Contracts to Strategic Partnerships
- 34.4. Integration / Adoption of New and Innovative Technologies
- 34.4.1. Single-use Bioreactors
- 34.4.2. Novel Bioprocess Techniques
- 34.4.3. Bioprocess Automation
- 34.5. Focus on Niche Therapeutic Areas
- 34.6. Growing Biosimilars Market to Contribute to the Growth of the Contract Services Segment
- 34.7. Capability Expansion by CMOs to become One-Stop-Shops
- 34.8. Offshoring Outsourcing Activities to Maximize Profits and Expand Existing Capacities
- 34.9. Increase in Financial Inflow and Outsourcing Budgets
- 34.10. Challenges Faced by Sponsors and Service Providers
- 34.10.1. Concerns Related to Single-use Systems
- 34.10.2. Issues Related to Capacity Fluctuations
- 34.11. Concluding Remarks
35. CONCLUSION
36. EXECUTIVE INSIGHTS
- 36.1. Chapter Overview
- 36.2. Company A
- 36.2.1. Company Snapshot
- 36.2.2. Interview Transcript: Chief Executive Officer
- 36.3. Company B
- 36.3.1. Company Snapshot
- 36.3.2. Interview Transcript: Chief Executive Officer And Co-Founder
- 36.4. Company C
- 36.4.1. Company Snapshot
- 36.4.2. Interview Transcript: Chief Technical Officer, Cell And Gene Therapy
- 36.5. Company D
- 36.5.1. Company Snapshot
- 36.5.2. Interview Transcript: President and Chief Operating Officer
- 36.6. Company E
- 36.6.1. Company Snapshot
- 36.6.2. Interview Transcript: Senior Director Of Global Strategic Marketing
- 36.7. Company F
- 36.7.1. Company Snapshot
- 36.7.2. Interview Transcript:Senior Director of Commercial Strategy and Market Insights
- 36.8. Company G
- 36.8.1. Company Snapshot
- 36.8.2. Interview Transcript:Global Head of Sales and Marketing and Head of Business Development (Germany)
- 36.9. Company H
- 36.9.1. Company Snapshot
- 36.9.2. Interview Transcript: Business Development Manager
- 36.10. Company I
- 36.10.1. Company Snapshot
- 36.10.2. Interview Transcript: Manager Marketing and Sales



















