
|
시장보고서
상품코드
1869576
마이크로바이옴 제조 시장 : 업계 동향과 세계 예측(-2035년) - 제조되는 제품 유형별, 제제별, 사용되는 1차 포장 유형별, 사업 규모별, 기업 규모별, 주요 지역별Microbiome Manufacturing Market: Industry Trends and Global Forecasts, Till 2035 - Distribution by Product Manufactured, Formulation, Primary Packaging Used, Scale of Operation, Company Size, Key Geographical Regions and Leading Developers |
||||||
마이크로바이옴 제조 시장 : 개요
Roots Analysis의 조사에 따르면, 세계 마이크로바이옴 제조 시장 규모는 현재 4,060만 달러에서 2035년까지 1억 8,670만 달러로 성장할 것으로 예상되며, 예측 기간(2035년까지) 동안 14.9%의 연평균 복합 성장률(CAGR)을 보일 것으로 예측됩니다.
시장 규모 및 기회 분석은 다음 매개 변수를 기반으로 분류됩니다.
생산되는 제품 유형
- API
- FDF
제형 유형
- 고체
- 액체
- 기타
사용되는 1차 포장 유형
- 블리스터 포장
- 유리/플라스틱 병
- 파우치/샤쉐
- 바이알
사업 규모
- 임상
- 상업
기업 규모
- 소규모
- 중규모
- 대기업 및 초대형 기업
주요 지역
- 북미
- 유럽
- 아시아태평양
- 기타 지역
마이크로바이옴 제조 시장 : 성장과 트렌드
인체에는 유익한 종과 유해한 종을 모두 포함하는 많은 미생물 군집이 존재하며, 이를 통칭하여 '미생물군집(마이크로바이옴)이라고 합니다. 마찬가지로 숙주 시스템 내에 서식하는 공생미생물, 공생미생물, 병원성 미생물의 생태계를 '마이크로바이옴'이라고 합니다. 미생물총이 질병의 발병과 병태생성에 미치는 영향을 고려할 때, 마이크로바이옴을 표적으로 하는 치료법의 개념은 의학 연구 커뮤니티의 주목을 받고 있습니다.
마이크로바이옴 기반 치료법은 비만, 당뇨병, 면역계 질환, 소화기 또는 위장 장애 등 다양한 질병에 대한 효과적이고 대안적인 치료 옵션을 제공합니다. 마이크로바이옴 기반 생수처리제(LBPs)는 박테리아, 효모 등 살아있는 미생물을 함유한 제제로 장내 마이크로바이옴을 회복, 조절, 강화하여 질병을 치료하거나 예방하는 것을 목적으로 합니다. 주목할 만한 점은 현재 전 세계적으로 165개 이상의 임상시험이 진행 중이며, 다양한 마이크로바이옴 치료 제품을 탐색하고 있다는 점입니다. 이러한 추세는 이해관계자들이 이 분야에서 대규모 개발 노력을 추진하고 있음을 보여줍니다.
그러나 마이크로바이옴 기반 치료제 생산은 개발 기간의 장기화, 엄격한 온도 관리 요건, 다양한 호기성/혐기성 균주(미생물 유형에 따라 다름)의 복잡한 엔지니어링, 최종 제품의 품질 특성 불균일성 등 몇 가지 복잡한 문제를 수반합니다. 생산 능력 부족도 장내 미생물 균주를 개발하는 기업들에게 우려되는 부분입니다. 지난 몇 년 동안 일부 기업들은 자체 제조 능력을 구축하여 이러한 문제를 해결했습니다. 그럼에도 불구하고, 새로운 자체 제조 시설 설립에 투자하기 전에 개념 증명 데이터를 원하는 기업도 여전히 존재합니다. 이러한 기업들은 현재 살아있는 마이크로바이옴 치료제의 제조를 CMO(위탁생산업체)에 위탁하고 있습니다. 이로 인해 이 분야에서 다양한 역량을 가진 소수의 수탁 제조업체에게 큰 기회가 생기고 있습니다. 마이크로바이옴 기반 제품의 제조 최적화를 위한 노력이 지속되고 있는 가운데, CMO 시장은 향후 10년간 꾸준한 성장이 예상됩니다.
마이크로바이옴 제조 시장 : 주요 연구 결과
이 보고서는 마이크로바이옴 제조 시장의 현황을 상세하게 분석하고 업계 내 잠재적인 성장 기회를 파악합니다. 주요 조사 결과는 다음과 같습니다.
- 현재 시장 상황에서는 20개 이상의 기존 기업과 신규 진출기업이 계약에 따라 생수처리제 제품 생산에 참여하고 있습니다.
- 이해관계자들은 다양한 규모의 비즈니스에 걸쳐 폭넓은 서비스를 제공하고 있다고 주장합니다. 완제의약품 중 비교적 많은 부분이 고체 및 액체 형태로 생산되고 있습니다.
- 미국에 자체 제조시설을 보유한 기업 중 대다수(50%)는 중견기업이며, 주로 전임상/임상 규모의 생산능력을 보유하고 있습니다.
- 지난 10년간 더 많은 기업들이 유럽에 제조 시설을 설립하는 추세를 보이고 있으며, 마이크로바이옴 제조 시장에서도 변화가 관찰되고 있습니다.
- 경쟁 우위를 확보하기 위해 업계 이해관계자들은 기존 역량을 적극적으로 강화하고, 마이크로바이옴에 특화된 서비스 포트폴리오를 확대하기 위해 노력하고 있습니다.

- 수요 증가에 대응하기 위해 많은 수탁 제조 기업들은 상호 이익이 되는 파트너십을 체결하거나 생산 능력을 확대하는 등 전략적인 노력을 기울이고 있습니다.
- 현재 각 지역에서 165개 이상의 임상시험(등록 환자 수 약 22,000명)이 진행 중이며, 마이크로바이옴 기반 치료제를 연구하고 있습니다.
- 마이크로바이옴 위탁생산 업체들은 앞으로도 마이크로바이옴 치료제 개발에 참여하는 기업들과 전략적 제휴를 맺고 해당 제품의 위탁생산을 지속할 것으로 예측됩니다.
- 전 세계 위탁생산 설비 용량은 다양한 지역에 분산되어 있지만, 흥미롭게도 전체 용량의 약 43%가 소규모 사업자가 소유한 시설에 설치되어 있습니다.
- 빠르게 확장되는 파이프라인과 효과적인 치료법에 대한 수요가 증가함에 따라, 마이크로바이옴 개발 기업들은 최종 제품의 고품질을 보장하기 위해 수탁 제조업체의 전문성을 활용하는 것을 선호하고 있습니다.

- 더 많은 개발업체들이 의약품 제조 업무의 다양한 측면을 아웃소싱함에 따라 향후 10년간 마이크로바이옴 위탁생산 시장은 연평균 14.9% 이상 성장할 것으로 예측됩니다.
마이크로바이옴 제조 시장 : 주요 부문
현재, API는 마이크로바이옴 제조 시장에서 가장 큰 점유율을 차지하고 있습니다.
제조되는 제품 유형별로 시장은 API(원료의약품)와 FDF(완제의약품)로 구분됩니다. 현재 API 부문이 마이크로바이옴 제조 시장에서 가장 큰 점유율을 차지하고 있습니다. 이러한 추세는 가까운 시일 내에 바뀔 가능성은 낮다고 볼 수 있습니다.
제제 유형별로 시장은 고형제제, 액상제제, 기타 제제로 구분됩니다. 현재, 액제가 마이크로바이옴 제조 시장에서 가장 큰 점유율을 차지하고 있습니다. 이러한 추세는 향후 몇 년 동안 지속될 것으로 보입니다.
1차 포장 유형별로 시장은 블리스 터 팩, 유리/플라스틱 병, 향낭/파우치, 바이알로 분류됩니다. 주목할 만한 점은 현재 마이크로바이옴 제조 시장에서 향낭/파우치가 더 큰 점유율을 차지하고 있다는 점입니다. 이러한 추세는 향후 10년간 지속될 것으로 예측됩니다.
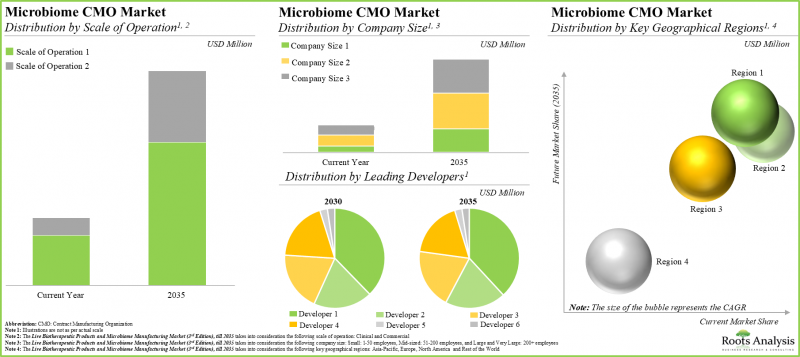
운영 규모에 따라 시장은 임상 규모와 상업 규모로 구분됩니다. 상업적 규모의 제조가 전체 시장의 주요 견인차 역할을 하는 반면, 임상 규모의 마이크로바이옴 제조 시장은 상대적으로 높은 CAGR로 성장할 가능성이 높다는 점에 주목해야 합니다.
기업 규모에 따라 시장은 소규모 기업, 중견기업, 대규모 및 초대형 기업으로 구분됩니다. 현재로서는 소규모 기업이 마이크로바이옴 제조 시장에서 가장 큰 수익을 창출하고 있습니다. 이러한 추세는 가까운 시일 내에 바뀔 가능성은 높지 않을 것으로 보입니다.
주요 지역별로 시장은 북미, 유럽, 아시아태평양 및 기타 지역으로 분류됩니다. 현재 마이크로바이옴 제조 시장의 대부분은 유럽이 차지하고 있습니다. 주목할 만한 점은 향후 몇 년 동안 세계 다른 지역 시장이 더 높은 CAGR로 성장할 것으로 예상된다는 점입니다.
마이크로바이옴 제조 시장을 대표하는 기업들
- Biose
- BJP Laboratories
- Capsugel
- Chr. Hansen
- Infant Bacterial Therapeutics
- Inpac Probiotics
- MaaT Pharma
- Microbiomik Healthcare
- NIZO
- OxThera
- Rebiotix
- Seres Therapeutics
- WACKER
- Winclove
목차
제1장 서문
제1장 생체 바이오의약품 및 마이크로바이옴 제조 시장 개요
제2장 조사 방법
제3장 경제적 및 기타 프로젝트 특유의 고려사항
제4장 주요 요약
제5장 서론
- 본 장의 개요
- 인간 미생물총과 마이크로바이옴의 개념
- Gut Flora 개요
- 인간 마이크로바이옴 프로제트(HMP)
- 마이크로바이옴 요법 개요
- 마이크로바이옴베이스 제품 제조
- 적절한 CMO 파트너를 선택할 때에 고려해야 할 중요점
제6장 시장 구도
- 본 장의 개요
- 생체 바이오의약품 및 마이크로바이옴 수탁 제조업체 : 시장 구도
- 생체 바이오의약품 및 마이크로바이옴 자사 제조업체 : 시장 구도
제7장 지역 능력 분석
제8장 기업 경쟁력 분석
제9장 기업 개요
- 본 장의 개요
- 북미의 생체 바이오의약품 및 마이크로바이옴 수탁 제조업체
- Capsugel
- 기타 기업
- Arranta Bio
- FUJIFILM Diosynth Biotechnologies
- List Biological Laboratories
- ProbioFerm
- 유럽의 생체 바이오의약품 및 마이크로바이옴 수탁 제조업체
- Biose Industrie
- Cerbios-Pharma
- Chr. Hansen
- Inpac Probiotics
- NIZO
- WACKER
- Winclove Probiotics
- 기타 기업
- BacThera
- Evologic Technologies
- Probiotical
- QUAY Pharma
- 아시아태평양의 생체 바이오의약품 및 마이크로바이옴 수탁 제조업체
- BJP Laboratories
- 기타 기업
- Aumgene Biosciences
- AcuraBio
- Meteoric Biopharmaceuticals
- Probiotics Australia
- Unique Biotech
제10장 파트너 후보 분석
제11장 대형 제약회사의 이니셔티브
제12장 최근 동향과 임해
제13장 임상시험 분석
제14장 용량 분석
제15장 수요 분석
제16장 제조 vs. 구입 의사결정 프레임워크
제17장 사례 연구 : 생바이오의약품과 마이크로바이옴 수탁연구기관(CRO) 및 식이보충제 제조업체
제18장 시장 영향 분석 : 성장 촉진요인 및 억제요인, 기회, 과제
- 본 장의 개요
- 시장 성장 촉진요인
- 시장 성장 억제요인
- 시장 기회
- 시장이 해결해야 할 과제
- 결론
제19장 생체 바이오의약품 및 마이크로바이옴 수탁 제조 시장
제20장 생체 바이오의약품 및 마이크로바이옴 수탁 제조 시장(제조되는 제품 유형별)
제21장 생체 바이오의약품 및 마이크로바이옴 수탁 제조 시장(제제 유형별)
제22장 생바이오의약품 및 마이크로바이옴 수탁 제조 시장(1차 포장 유형별)
제23장 생체 바이오의약품 및 마이크로바이옴 수탁 제조 시장(사업 규모별)
제24장 생체 바이오의약품 및 마이크로바이옴 수탁 제조 시장(기업 규모별)
제25장 생체 바이오의약품 및 마이크로바이옴 수탁 제조 시장(주요 지역별)
제26장 생체 바이오의약품 및 마이크로바이옴 요법 주요 개발자
제27장 경영진 인사이트
제28장 결론
제29장 부록 I : 표 데이터
제30장 부록 II : 기업 및 조직 리스트
LSH 25.11.24Microbiome Manufacturing Market: Overview
As per Roots Analysis, the global microbiome manufacturing market is estimated to grow from USD 40.6 million in the current year to USD 186.7 million by 2035, at a CAGR of 14.9% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Product Manufactured
- API
- FDF
Type of Formulation
- Solid
- Liquid
- Others
Type of Primary Packaging Used
- Blister Packs
- Glass / Plastic Bottles
- Pouches / Sachets
- Vials
Scale of Operation
- Clinical
- Commercial
Company Size
- Small
- Mid-sized
- Large and Very Large
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
Microbiome Manufacturing Market: Growth and Trends
The human body contains many communities of microbes, encompassing both advantageous and harmful species, which are collectively called microbiota. Similarly, the ecological system of commensal, symbiotic, and pathogenic microorganisms living within a host system is referred to as the microbiome. Considering the influence of microbiota on disease development and pathogenesis, the concept of microbiome-targeted therapies has gradually captivated the attention of the medical research communities.
Microbiome based therapeutics provide an effective and alternative treatment option for a range of conditions, such as obesity, diabetes, immune system disorders, and digestive or gastrointestinal disorders. Microbiome-based live biotherapeutic products (LBPs) are formulations containing live microorganisms, such as bacteria or yeasts, designed to restore, modulate, or enhance the gut microbiome to treat or prevent diseases. It is worth highlighting that more than 165 clinical trials are currently underway to explore various microbiome-based therapeutic products across diverse regions. This trend is indicative of the extensive development efforts being undertaken by stakeholders in this domain.
However, microbiome-based therapeutics production is associated with several complexities, including prolonged development timelines, stringent temperature control requirements, complex engineering of diverse aerobic / anaerobic strains (depending on the type of microbe) and inconsistencies related to quality attributes of the final product. The lack of manufacturing capacity is another concern for companies developing intestinal microbiome strains. In the past few years, some players have circumvented this challenges by establishing in-house manufacturing capabilities. Nonetheless, some companies still require proof-of-concept data before investing in the establishment of a new in-house manufacturing facility. Such companies are currently outsourcing their operations to CMOs for live microbiome therapies. This provides significant opportunities for a few contract manufacturers with varying capabilities in this domain. With the ongoing pace of efforts to optimize the manufacturing of microbiome-based products, the CMO market is anticipated to witness steady growth in the coming decade.
Microbiome Manufacturing Market: Key Insights
The report delves into the current state of the microbiome manufacturing market and identifies potential growth opportunities within industry. Some key findings from the report include:
- The current market landscape features the presence of over 20 well-established players and new entrants that are engaged in the production of live biotherapeutics products, on contractual basis.
- Stakeholders claim to offer a range of services across different scales of operation; a relatively larger proportion of the finished drug products are manufactured in solid and liquid forms.
- Majority (50%) of the players having in-house manufacturing facilities in the US are mid-sized; these firms primarily have capabilities for pre-clinical / clinical scale production.
- In the past decade, a shift in trend has been observed in the microbiome manufacturing market as more players have set up manufacturing facilities in Europe.
- In pursuit of gaining a competitive edge, industry stakeholders are actively upgrading existing capabilities and enhancing their microbiome-focused service portfolios.
- To keep pace with the growing demand, many contract manufacturers have undertaken strategic initiatives, such as entering into mutually beneficial partnerships and expanding their capacities.
- More than 165 clinical trials (with nearly 22,000 enrolled patients) are currently underway to investigate microbiome based-therapeutic products, across different geographies.
- Microbiome contract manufacturers are anticipated to continue to form strategic alliances with players engaged in the development of microbiome therapeutics for contract manufacturing of their products.
- The global, installed contract manufacturing capacity is spread across various geographies; interestingly, around 43% of the total capacity is installed at the facilities owned by small players.
- Given the rapidly growing pipeline and the increasing demand for effective therapeutic interventions, microbiome developers prefer to leverage the expertise of contract manufacturers to ensure high quality of end-products.
- As more developers outsource various aspects of their respective drug manufacturing operations, it is anticipated that the microbiome CMOs market to grow at an annualized rate of over 14.9% in the coming decade.
Microbiome Manufacturing Market: Key Segments
Currently, API Occupies the Largest Share of the Microbiome Manufacturing Market
Based on the type of product manufactured, the market is segmented into API and FDF. At present, the API segment holds the maximum share of the microbiome manufacturing market. This trend is unlikely to change in the near future.
Currently, Liquid Formulation Segment Accounts for the Largest Share of the Microbiome Manufacturing Market
Based on the type of formulation, the market is segmented into solid, liquid and other formulations. Currently, liquid formulation holds the maximum share of the microbiome manufacturing market. This trend is likely to remain the same in the forthcoming years.
Sachets / Pouches is the Fastest Growing Segment in the Microbiome Manufacturing Market During the Forecast Period
Based on the type of primary packaging used, the market is segmented into blister packs, glass / plastic bottles, sachets / pouches and vials. It is worth highlighting that, at present, sachets / pouches hold a larger share of the microbiome manufacturing market. This trend is likely to remain the same in the coming decade.
By Scale of Operation, Commercial Scale is Likely to Dominate the Microbiome Manufacturing Market During the Forecast Period
Based on the scale of operation, the market is segmented into clinical and commercial scales. Whilst commercial scale manufacturing will be the primary driver of the overall market, it is worth highlighting that the microbiome manufacturing market at the clinical scale is likely to grow at a relatively higher CAGR.
Currently, Small Companies Occupies the Largest Share of the Microbiome Manufacturing Market
Based on the company size, the market is segmented into small, mid-sized, and large and very large companies. At present, small companies generate maximum revenue of the microbiome manufacturing market. This trend is unlikely to change in the near future.
Europe Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific and Rest of the world. Majority of the current microbiome manufacturing market is captured by Europe. It is worth highlighting that the market in the rest of the world is expected to grow at a higher CAGR in the coming years.
Example Players in the Microbiome Manufacturing Market
- Biose
- BJP Laboratories
- Capsugel
- Chr. Hansen
- Infant Bacterial Therapeutics
- Inpac Probiotics
- MaaT Pharma
- Microbiomik Healthcare
- NIZO
- OxThera
- Rebiotix
- Seres Therapeutics
- WACKER
- Winclove
Primary Research Overview
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:
- Founder and Executive Chairman, Company A
- Managing Director and Scientific Head, Company B
- Managing Director and Chief Executive Officer, Company C
- Co- Founder and Chief Executive Officer, Company D
- Founder and Chief Executive Officer, Company E
- Chief Business Officer, Company F
- Chief Operating Officer, Company G
- Former Vice President, Business Development, Company H
- Former Vice President, Commercial Operations, Company I
- Former Vice President, Business Development, Company J
- Former Vice President. Sales and Business Development, Company K
- Head of Business Development, Company L
- Business Development Manager, Company M
Microbiome Manufacturing Market: Research Coverage
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the microbiome manufacturing market, focusing on key market segments, including [A] type of product manufactured, [B] type of formulation, [C] type of primary packaging used, [D] scale of operation, [E] company size and [F] key geographical regions.
- Market Landscape: A comprehensive evaluation of microbiome manufacturing service providers, considering various parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] scale of operation, [E] type of product manufactured, [F] type of formulation, [G] type of primary packaging used, [H] type of microbe used, [I] type of service offered, [J] number and location of manufacturing facilities and bioprocessing capacity, and [K] type of microbial species used.
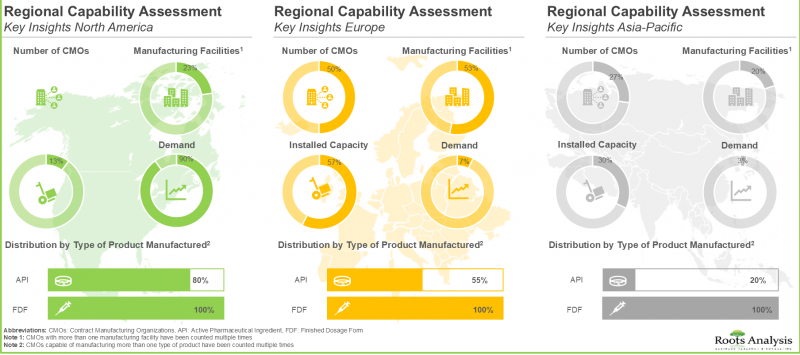
- Regional Capability Analysis: A detailed landscape of the microbiome manufacturing facilities established across key geographical regions (North America, Europe and Asia-Pacific), highlighting the key manufacturing hubs for microbiomes.
- Company Competitiveness Analysis: A comprehensive competitive analysis of microbiome manufacturing service providers, examining factors, such as [A] supplier strength [B] portfolio diversity and [C] number of services offered.
- Company Profiles: In-depth profiles of key microbiome manufacturing service providers offering contract services for live biotherapeutic products at both clinical and commercial scale, focusing on [A] company overviews, [B] microbiome-related service portfolio, [C] microbiome manufacturing facilities, [D] recent developments and [E] an informed future outlook.
- Likely Partner Analysis: A detailed evaluation of nearly 70 microbiome-focused drug developers that are most likely to collaborate with contract manufacturers. This analysis considers various relevant parameters, including [A] developer strength (in terms of company size and its experience), [B] pipeline maturity (which takes into account the number of pipeline drugs and their affiliated stage of development), [C] pipeline strength (which takes into account the number of microbiome drugs in the company's pipeline), and [D] availability of in-house manufacturing capabilities.
- Big Pharma Analysis: A comprehensive examination of various microbiome-focused initiatives undertaken by major pharmaceutical companies. This analysis includes heat map visualizations that illustrate the distribution of leading pharmaceutical firms, as well as spider web diagrams that compare their initiatives across multiple relevant parameters.
- Recent Development and Initiatives: An analysis of recent trends, covering partnerships and collaborations, mergers and acquisitions, and expansion initiatives held in this domain.
- Clinical Trials Analysis: Examination of completed, ongoing, and planned clinical studies of various microbiome therapeutics, based on parameters, such as [A] trial registration year, [B] trial status, [C] trial phase, [D] enrolled patient population, [E] type of sponsor, [F] most active industry players (in terms of number of trials conducted), [G] study design, [H] therapeutic area and [I] key geographical regions.
- Capacity Analysis: Estimation of global microbiome-based therapies manufacturing capacity, derived from data provided by various industry stakeholders in the public domain. This analysis emphasizes the distribution of the available capacity on the basis of company size (small, mid-sized, and large and very large) and key geographical regions (North America, Europe, Asia-Pacific and Rest of the World).
- Demand Analysis: Informed estimates of the annual commercial and clinical demand for microbiome therapeutics, taking into account the target patient population in ongoing and planned clinical trials of microbiome therapeutics, sponsored by both industry and non-industry players.
- Make Versus Buy Decision Making Framework: An insightful framework that emphasizes the key indicators and factors that need to be considered by microbiome therapeutics developers to determine whether to manufacture their respective products in-house or outsource the manufacturing operation to contract service providers.
- Case Study: A case study on the current market landscape of microbiome contract research organizations and dietary supplement providers, including information on their year of establishment, company size and location of headquarters.
Key Questions Answered in this Report
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What is the current global capacity of microbiome manufacturers?
- Which microbiome therapy developers are most likely to collaborate with service providers?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
Reasons to Buy this Report
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
Additional Benefits
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
1.1. Live Biotherapeutic Products and Microbiome Manufacturing Market Overview
- 1.2. Key Market Insights
- 1.3. Scope of the Report
- 1.4. Inclusions and Exclusions
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.3. Project Methodology
- 2.4. Forecast Methodology
- 2.5. Robust Quality Control
- 2.6. Key Market Segmentations
- 2.7. Key Considerations
- 2.7.1. Demographics
- 2.7.2. Economic Factors
- 2.7.3. Government Regulations
- 2.7.4. Supply Chain
- 2.7.5. COVID Impact / Related Factors
- 2.7.6. Market Access
- 2.7.7. Healthcare Policies
- 2.7.8. Industry Consolidation
3. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Future Estimates
- 3.2.2. Currency Coverage
- 3.2.2.1. Overview of Major Currencies Affecting the Market
- 3.2.2.2. Impact of Currency Fluctuations on the Industry
- 3.2.3. Foreign Exchange Impact
- 3.2.3.1. Evaluation of Foreign Exchange Rates and Their Impact on Market
- 3.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. EXECUTIVE SUMMARY
5. INTRODUCTION
- 5.1. Chapter Overview
- 5.2. Concept of Human Microbiota and Microbiome
- 5.3. Overview of Gut Flora
- 5.3.1. Role of Gut Flora in Healthy Individuals
- 5.3.2. Factors Affecting Human Gut Flora
- 5.4. The Human Microbiome Project (HMP)
- 5.5. Overview of Microbiome Therapies
- 5.5.1. Types of Microbiome Therapies
- 5.5.1.1. Probiotics
- 5.5.1.2. Prebiotics
- 5.5.2. Applications of Microbiome Therapies
- 5.5.3. Microbiome Therapies Supply Chain
- 5.5.1. Types of Microbiome Therapies
- 5.6. Microbiome-based Product Manufacturing
- 5.6.1. Key Steps Involved
- 5.6.2. Challenges Associated with Manufacturing of Microbiome Therapeutics
- 5.6.3. Role of Contract Manufacturing Organizations (CMOs)
- 5.6.4. Demand for Contract Manufacturing Services
- 5.7. Key Considerations while Selecting a Suitable CMO Partner
6. MARKET LANDSCAPE
- 6.1. Chapter Overview
- 6.2. Live Biotherapeutic Products and Microbiome Contract Manufacturers: Market Landscape
- 6.2.1. Analysis by Year of Establishment
- 6.2.2. Analysis by Company Size
- 6.2.3. Analysis by Location of Headquarters
- 6.2.4. Analysis by Scale of Operation
- 6.2.5. Analysis by Type of Product Manufactured
- 6.2.6. Analysis by Type of Formulation
- 6.2.7. Analysis by Scale of Operation and Type of Formulation
- 6.2.8. Analysis by Type of Primary Packaging Used
- 6.2.9. Analysis by Type of Formulation and Type of Primary Packaging Used
- 6.2.10. Analysis by Type of Microbe Used
- 6.2.11. Analysis by Type of Microbe Used and Location of Headquarters
- 6.2.12. Analysis by Type of Service Offered
- 6.2.13. Analysis by Number of Manufacturing Facilities (Region)
- 6.2.14. Analysis by Number of Manufacturing Facilities
(Country)
- 6.2.15. Analysis by Type of Microbial Species Used
- 6.3. Live Biotherapeutic Products and Microbiome In-House Manufacturers: Market Landscape
- 6.3.1. Analysis by Year of Establishment
- 6.3.2. Analysis by Company Size
- 6.3.3. Analysis by Location of Headquarters
- 6.3.4. Analysis by Scale of Operation
- 6.3.5. Analysis by Location of Manufacturing Facilities
7. REGIONAL CAPABILITY ANALYSIS
- 7.1. Chapter Overview
- 7.2. Key Assumptions and Parameters
- 7.3. Live Biotherapeutic Products and Microbiome Contract Manufacturers in North America
- 7.4. Live Biotherapeutic Products and Microbiome Contract Manufacturers in Europe
- 7.5. Live Biotherapeutic Products and Microbiome Contract Manufacturers in Asia-Pacific
8. COMPANY COMPETITIVENESS ANALYSIS
- 8.1. Chapter Overview
- 8.2. Key Assumptions and Parameters
- 8.3. Methodology
- 8.4. Company Competitiveness Analysis: Live Biotherapeutic Products and Microbiome Manufacturers
- 8.5. Capability Benchmarking of Top Microbiome Contract Manufactures
9. COMPANY PROFILES
- 9.1. Chapter Overview
- 9.2. Leading Live Biotherapeutic Products and Microbiome Contract Manufacturers in North America
- 9.2.1. Capsugel
- 9.2.1.1. Company Overview
- 9.2.1.2. Service Portfolio
- 9.2.1.3. Facilities Dedicated to Microbiome Manufacturing
- 9.2.1.4. Recent Developments and Future Outlook
- 9.2.1. Capsugel
- 9.3. Other Prominent Live Biotherapeutic Products and Microbiome Contract Manufacturers in North America
- 9.3.1. Arranta Bio
- 9.3.1.1. Company Overview
- 9.3.1.2. Service Portfolio
- 9.3.2. FUJIFILM Diosynth Biotechnologies
- 9.3.2.1. Company Overview
- 9.3.2.2. Service Portfolio
- 9.3.3. List Biological Laboratories
- 9.3.3.1. Company Overview
- 9.3.3.2. Service Portfolio
- 9.3.4. ProbioFerm
- 9.3.4.1. Company Overview
- 9.3.4.2. Service Portfolio
- 9.3.1. Arranta Bio
- 9.4. Leading Live Biotherapeutic Products and Microbiome Contract Manufacturers in Europe
- 9.4.1. Biose Industrie
- 9.4.1.1. Company Overview
- 9.4.1.2. Service Portfolio
- 9.4.1.3. Facilities Dedicated to Microbiome Manufacturing
- 9.4.1.4. Recent Developments and Future Outlook
- 9.4.2. Cerbios-Pharma
- 9.4.2.1. Company Overview
- 9.4.2.2. Service Portfolio
- 9.4.2.3. Facilities Dedicated to Microbiome Manufacturing
- 9.4.2.4. Recent Developments and Future Outlook
- 9.4.3. Chr. Hansen
- 9.4.3.1. Company Overview
- 9.4.3.2. Financial Information
- 9.4.3.3. Service Portfolio
- 9.4.3.4. Facilities Dedicated to Microbiome Manufacturing
- 9.4.3.5. Recent Developments and Future Outlook
- 9.4.4. Inpac Probiotics
- 9.4.4.1. Company Overview
- 9.4.4.2. Service Portfolio
- 9.4.4.3. Facilities Dedicated to Microbiome Manufacturing
- 9.4.4.4. Recent Developments and Future Outlook
- 9.4.5. NIZO
- 9.4.5.1. Company Overview
- 9.4.5.2. Service Portfolio
- 9.4.5.3. Facilities Dedicated to Microbiome Manufacturing
- 9.4.5.4. Recent Developments and Future Outlook
- 9.4.6. WACKER
- 9.4.6.1. Company Overview
- 9.4.6.2. Service Portfolio
- 9.4.6.3. Facilities Dedicated to Microbiome Manufacturing
- 9.4.6.4. Recent Developments and Future Outlook
- 9.4.7 Winclove Probiotics
- 9.4.7.1. Company Overview
- 9.4.7.2. Service Portfolio
- 9.4.7.3. Facilities Dedicated to Microbiome Manufacturing
- 9.4.7.4. Recent Developments and Future Outlook
- 9.4.1. Biose Industrie
- 9.5. Other Prominent Live Biotherapeutic Products and Microbiome Contract Manufacturers in Europe
- 9.5.1. BacThera
- 9.5.1.1. Company Overview
- 9.5.1.2. Service Portfolio
- 9.5.2. Evologic Technologies
- 9.5.2.1 Company Overview
- 9.5.2.2. Service Portfolio
- 9.5.3. Probiotical
- 9.5.3.1. Company Overview
- 9.5.3.2. Service Portfolio
- 9.5.4. QUAY Pharma
- 9.5.4.1. Company Overview
- 9.5.4.2. Service Portfolio
- 9.5.1. BacThera
- 9.6. Leading Live Biotherapeutic Products and Microbiome Contract Manufacturers in Asia-Pacific
- 9.6.1. BJP Laboratories
- 9.6.1.1. Company Overview
- 9.6.1.2. Service Portfolio
- 9.6.1.3. Facilities Dedicated to Microbiome Manufacturing
- 9.6.1.4. Recent Developments and Future Outlook
- 9.6.1. BJP Laboratories
- 9.7. Other Prominent Live Biotherapeutic Products and Microbiome Contract Manufacturers in Asia-Pacific
- 9.7.1. Aumgene Biosciences
- 9.7.1.1 Company Overview
- 9.7.1.2. Service Portfolio
- 9.7.2. AcuraBio
- 9.7.2.1. Company Overview
- 9.7.2.2. Service Portfolio
- 9.7.3. Meteoric Biopharmaceuticals
- 9.7.3.1. Company Overview
- 9.7.3.2. Service Portfolio
- 9.7.4. Probiotics Australia
- 9.7.4.1. Company Overview
- 9.7.4.2. Service Portfolio
- 9.7.5. Unique Biotech
- 9.7.5.1 Company Overview
- 9.7.5.2. Service Portfolio
- 9.7.1. Aumgene Biosciences
10. LIKELY PARTNER ANALYSIS
- 10.1. Chapter Overview
- 10.2. Key Assumptions and Parameters
- 10.3. Methodology
- 10.4. Key Potential Strategic Partners for Live Biotherapeutic Products and Microbiome Contract Manufacturers
- 10.4.1. Likely Partners in North America
- 10.4.2. Likely Partners in Europe
- 10.4.3. Likely Partners in Asia-Pacific
11. BIG PHARMA INITIATIVES
- 11.1. Chapter Overview
- 11.2. Methodology
- 11.3. Live Biotherapeutic Products and Microbiome-related Initiatives of Big Pharmaceutical Players
- 11.3.1. Analysis by Portfolio Diversity
- 11.3.2. Analysis by Trial Phase
- 11.3.3. Analysis by Type of Therapy
- 11.3.4. Analysis by Type of Molecule
- 11.3.5. Analysis by Therapeutic Area
- 11.3.6. Analysis by Number of Partnership and Funding Instances
- 11.4. Benchmark Analysis of Big Pharmaceutical Players
- 11.4.1. Spider Web Analysis: Early Stage / Late Stage Products
- 11.4.2. Spider Web Analysis: Portfolio Diversity
- 11.4.3. Spider Web Analysis: Funding and Investments
- 11.4.4. Spider Web Analysis: Partnerships and Collaborations
- 11.4.5. Spider Web Analysis: Therapeutic Areas
12. RECENT DEVELOPMENTS AND INITIATIVES
- 12.1. Chapter Overview
- 12.2. Partnerships and Collaborations
- 12.2.1. Partnership Models
- 12.2.2. Live Biotherapeutic Products and Microbiome Manufacturing: List of Partnerships and Collaborations
- 12.2.3. Analysis by Year of Partnership
- 12.2.4. Analysis by Type of Partnership
- 12.2.5. Analysis by Year and Type of Partnership
- 12.2.6. Analysis by Type of Organization
- 12.2.7. Analysis by Type of Partnership and Type of Organization
- 12.2.8. Analysis by Therapeutic Area
- 12.2.9. Most Active Players: Analysis by Number of Partnerships
- 12.2.9. Analysis by Geography
- 12.2.9.1. Intracontinental and Intercontinental Agreements
- 12.2.9.2. Local and International Agreements
- 12.3. Cumulative Year-wise Trend of Merger / Acquisition
- 12.3.1. Analysis by Key Value Drivers
- 12.3.2. Analysis by Year of Acquisition and Key Value Drivers
- 12.4. Recent Expansions
- 12.4.1. Purpose of Expansions
- 12.4.2. Live Biotherapeutic Products and Microbiome Manufacturing: Recent Expansions
- 12.4.1. Analysis by Year of Expansion
- 12.4.2. Analysis by Purpose of Expansion
- 12.4.3. Analysis by Year and Purpose of Expansion
- 12.4.4. Analysis by Scale of Operation
- 12.4.5. Analysis by Purpose of Expansion and Scale of Operation
- 12.4.6. Analysis by Geography
- 12.4.6.1. Analysis by Location of Expanded Facility (Region)
- 12.4.6.2. Analysis by Location of Expanded Facility (Country)
- 12.4.7. Analysis by Purpose of Expansion and Location of Expanded Facility (Region)
13. CLINICAL TRIAL ANALYSIS
- 13.1. Chapter Overview
- 13.2. Scope and Methodology
- 13.3. Live Biotherapeutic Products and Microbiome Manufacturing: Clinical Trial Analysis
- 13.3.1. Analysis by Trial Registration Year
- 13.3.2. Analysis by Trial Status
- 13.3.3. Analysis by Trial Registration Year and Trial Status
- 13.3.4. Analysis by Trial Phase
- 13.3.5. Analysis by Trial Registration Year and Trial Phase
- 13.3.6. Analysis of Enrolled Patient Population by Trial Registration Year
- 13.3.7. Analysis of Enrolled Patient Population by Trial Phase
- 13.3.8. Analysis by Type of Sponsor
- 13.3.9. Most Active Players: Analysis by Number of Registered Trials
- 13.3.10. Analysis by Study Design
- 13.3.11. Analysis by Therapeutic Area
- 13.3.12. Analysis of Number of Registered Trials by Geography
- 13.3.13. Analysis of Number of Registered Trials by Trial Status and Geography
- 13.3.14. Analysis of Enrolled Patient Population by Geography
- 13.3.15. Analysis of Enrolled Patient Population by Trial Status and Geography
14. CAPACITY ANALYSIS
- 14.1. Chapter Overview
- 14.2. Key Assumptions
- 14.3. Methodology
- 14.4. Live Biotherapeutic Products and Microbiome Contract Manufacturing: Global Installed Capacity
- 14.4.1. Analysis by Company Size
- 14.4.2. Analysis by Geography
- 14.4.2.1. Analysis of Installed Live Biotherapeutic Products and Microbiome Contract Manufacturing Capacity in North America
- 14.4.2.2. Analysis of Installed Live Biotherapeutic Products and Microbiome Contract Manufacturing Capacity in Europe
- 14.4.2.3. Analysis of Installed Live Biotherapeutic Products and Microbiome Contract Manufacturing Capacity Asia-Pacific and Rest of the World
15. DEMAND ANALYSIS
- 15.1. Chapter Overview
- 15.2. Methodology
- 15.3. Global Clinical Demand for Live Biotherapeutic Products and Microbiome Manufacturing
- 15.3.1. Analysis by Number of Trials Conducted
- 15.3.2. Analysis by Enrolled Patient Population
- 15.3.3. Analysis by Trial Phase
- 15.3.4. Analysis by Geography
- 15.3.4.1. Clinical Demand in North America
- 15.3.4.2. Clinical Demand in Europe
- 15.3.4.3. Clinical Demand in Asia-Pacific and Rest of the World
- 15.4. Global Commercial Demand for Live Biotherapeutic Products and Microbiome Manufacturing
16. MAKE VERSUS BUY DECISION MAKING FRAMEWORK
- 16.1. Chapter Overview
- 16.2. Key Assumptions and Parameters
- 16.3. Live Biotherapeutic Products and Microbiome Contract Manufacturers: Make versus Buy Decision Making Framework
- 16.3.1. Scenario 1
- 16.3.2. Scenario 2
- 16.3.3. Scenario 3
- 16.3.4. Scenario 4
- 16.4. Concluding Remarks
17. CASE STUDY: LIVE BIOTHERAPEUTIC PRODUCTS AND MICROBIOME CONTRACT RESEARCH ORGANIZATIONS (CROs) AND DIETARY SUPPLEMENT MANUFACTURERS
- 17.1 Chapter Overview
- 17.2. Live Biotherapeutic Products and Microbiome CROs
- 17.3. Live Biotherapeutic Products and Microbiome CROs: Market Landscape
- 17.3.1. Analysis by Year of Establishment
- 17.3.2. Analysis by Company Size
- 17.3.3. Analysis by Location of Headquarters
- 17.4. Microbiome Dietary Supplement Manufacturers: Market Landscape
- 17.4.1. Analysis by Year of Establishment
- 17.4.2. Analysis by Company Size
- 17.4.3. Analysis by Location of Headquarters
18. MARKET IMPACT ANALYSIS: DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES
- 18.1. Chapter Overview
- 18.2. Market Drivers
- 18.3. Market Restraints
- 18.4. Market Opportunities
- 18.5. Market Challenges
- 18.6. Conclusion
19. LIVE BIOTHERAPEUTIC PRODUCTS AND MICROBIOME CONTRACT MANUFACTURING MARKET
- 19.1. Chapter Overview
- 19.2. Key Assumptions and Methodology
- 19.3. Global Live Biotherapeutic Products and Microbiome Contract Manufacturing Market, till 2035
- 19.4. Scenario Analysis
- 19.4.1. Conservative Scenario
- 19.4.2. Optimistic Scenario
- 19.5. Key Market Segmentations
20. LIVE BIOTHERAPEUTIC PRODUCTS AND MICROBIOME CONTRACT MANUFACTURING MARKET, BY TYPE OF PRODUCT MANUFACTURED
- 20.1. Chapter Overview
- 20.2. Key Assumptions and Methodology
- 20.3. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market: Distribution by Type of Product Manufactured, Current Year and 2035
- 20.3.1. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for APIs, till 2035
- 20.3.2. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for FDFs, till 2035
- 20.4. Data Triangulation
21. LIVE BIOTHERAPEUTIC PRODUCTS AND MICROBIOME CONTRACT MANUFACTURING MARKET, BY TYPE OF FORMULATION
- 21.1. Chapter Overview
- 21.2. Key Assumptions and Methodology
- 21.3. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market: Distribution by Type of Formulation, Current Year and 2035
- 21.3.1. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Solids, till 2035
- 21.3.2. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Liquids, till 2035
- 21.3.3. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Others, till 2035
- 21.4. Data Triangulation
22. LIVE BIOTHERAPEUTIC PRODUCTS AND MICROBIOME CONTRACT MANUFACTURING MARKET, BY TYPE OF PRIMARY PACKAGING USED
- 22.1. Chapter Overview
- 22.2. Key Assumptions and Methodology
- 22.3. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market: Distribution by Type of Primary Packaging Used, Current Year and 2035
- 22.3.1. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Blister Packs, till 2035
- 22.3.2. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Glass / Plastic Bottles, till 2035
- 22.3.3. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Pouches / Sachets, till 2035
- 22.3.4. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Vials, till 2035
- 22.4. Data Triangulation
23. LIVE BIOTHERAPEUTIC PRODUCTS AND MICROBIOME CONTRACT MANUFACTURING MARKET, BY SCALE OF OPERATION
- 23.1. Chapter Overview
- 23.2. Key Assumptions and Methodology
- 23.3. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market: Distribution by Type of Scale of Operation, Current Year and 2035
- 23.3.1. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Clinical, till 2035
- 23.3.2. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Commercial, till 2035
- 23.4. Data Triangulation
24. LIVE BIOTHERAPEUTIC PRODUCTS AND MICROBIOME CONTRACT MANUFACTURING MARKET, BY COMPANY SIZE
- 24.1. Chapter Overview
- 24.2. Key Assumptions and Methodology
- 24.3. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market: Distribution by Company Size, Current Year and 2035
- 24.3.1. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Small Companies, till 2035
- 24.3.2. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Mid-Sized Companies, till 2035
- 24.3.3. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Large and Very Large Companies, till 2035
- 24.4. Data Triangulation
25. LIVE BIOTHERAPEUTIC PRODUCTS AND MICROBIOME CONTRACT MANUFACTURING MARKET, BY KEY GEOGRAPHICAL REGIONS
- 25.1. Chapter Overview
- 25.2. Key Assumptions and Methodology
- 25.3. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market: Distribution by Key Geographical Regions, Current Year and 2035
- 25.3.1. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for North America, till 2035
- 25.3.2. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Europe, till 2035
- 25.3.3. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Asia-Pacific, till 2035
- 25.3.4. Live Biotherapeutic Products and Microbiome Contract Manufacturing Market for Rest of the World, till 2035
- 25.4. Data Triangulation
26. LEADING DEVELOPERS FOR LIVE BIOTHERAPEUTIC PRODUCTS AND MICROBIOME THERAPIES
- 26.1. Chapter Overview
- 26.2. Key Assumptions and Methodology
- 26.3. Live Biotherapeutic Products and Microbiome Therapeutics Market: Distribution by Leading Developers
- 26.4. Data Triangulation
27. EXECUTIVE INSIGHTS
- 27.1. Chapter Overview
- 27.2. Company A
- 27.2.1. Company Snapshot
- 27.2.2. Interview Transcript: Founder and Executive Chairman
- 27.3. Company B
- 27.3.1. Company Snapshot
- 27.3.2. Interview Transcript: Managing Director and Scientific Head
- 27.4. Company C
- 27.4.1. Company Snapshot
- 27.4.2. Interview Transcript: Managing Director and Chief Executive Officer
- 27.5. Company D
- 27.5.1. Company Snapshot
- 27.5.2. Interview Transcript: Co- Founder and Chief Executive Officer
- 27.6. Company E
- 27.6.1. Company Snapshot
- 27.6.2. Interview Transcript: Founder and Chief Executive Officer
- 27.7. Company F
- 27.7.1. Company Snapshot
- 27.8.2. Interview Transcript: Chief Business Officer
- 27.8. Company G
- 27.8.1. Company Snapshot
- 27.8.2. Interview Transcript: Chief Operating Officer
- 27.9. Company H
- 27.9.1. Company Snapshot
- 27.9.2. Interview Transcript: Vice President, Business Development
- 27.10. Company I
- 27.10.1. Company Snapshot
- 27.10.2. Interview Transcript: Vice President, Commercial Operations
- 27.11. Company J
- 27.11.1. Company Snapshot
- 27.11.2. Interview Transcript: Vice President, Business Development
- 27.12. Company K
- 27.12.1. Company Snapshot
- 27.12.2. Interview Transcript: Vice President. Sales and Business Development
- 27.13. Company L
- 27.13.1. Company Snapshot
- 27.13.2. Interview Transcript: Head of Business Development
- 27.14. Company M
- 27.14.1. Company Snapshot
- 27.14.2. Interview Transcript: Business Development Manager



















