
|
시장보고서
상품코드
1891247
임상시험 지원기관 시장 : 치료영역별, 시험단계별, 임상시험 구성요소별, 개입유형별, 주요 지역별 업계 동향과 예측Site Management Organization Market: Industry Trends and Global Forecasts, Till 2035 - Distribution by Therapeutic Area, Trial Phases, Clinical Trial Components, Type of Interventions and Key Geographies |
||||||
임상시험 지원기관 시장 : 시장 개요
임상시험 지원기관 시장 규모는 올해 124억 달러에서 2035년까지 316억 달러로 성장하고 예측기간(2035년까지)의 CAGR은 9.8%를 나타낼 것으로 추정되고 있습니다.
임상시험 지원기관 시장 : 성장과 동향
임상시험은 의약품 개발 과정 전반에 걸쳐 매우 중요한 단계이며 후보 약물의 안전성과 효능을 평가하는 데 필수적인 역할을 담당합니다. 설문조사에 따르면 의약품 후보의 진행에 할당된 총 투자액의 약 40%가 임상시험에 지출되고 있으며 연간 비용은 780억 달러에 이릅니다. 그러나 이러한 시험의 실행에는 과학적 및 운영 상의 복잡성, 적절한 환자의 모집 및 유지에 관한 과제, 데이터 관리와 관련된 과제, 엄격한 규제 기준 등 심각한 과제가 종종 수반됩니다.
게다가 프로세스 전체에 내재된 복잡성과 여러 이해관계자의 참여로 인해 이러한 테스트는 지연이 발생하기 쉽습니다. 80% 이상의 임상시험이 1개월에서 6개월의 지연을 겪는 반면 예정대로 완료되는 시험은 10%에 불과합니다. 이러한 이유로 제약 업계의 혁신자들은 임상시험을 수행하는 방법과 효과적인 관리 기법을 개선하기 위해 지속적으로 노력하고 있습니다. 수많은 옵션 중에서도 임상시험 지원기관(SMO)과 같은 전문 서비스 제공업체에 다양한 시험 업무를 위탁하는 것은 많은 제약기업들에게 유력한 선택이 되고 있습니다. 복잡성이 증가하고 등록 임상시험 수가 증가함에 따라 SMO 수요는 예측기간을 통해 꾸준한 시장 성장으로 이어질 것으로 예상됩니다.
시장 성장 촉진요인
임상시험 지원기관(SMO) 시장은 임상시험의 건수와 복잡성 증가에 의해 견인되고 있습니다. 등록되는 연구에서는 2상 및 3상 시험이나 정밀의료에 있어서 전문적인 시험 지원이 필요하기 때문입니다. 또한 만성질환과 희소질환의 유병률 증가에 따라 전자건강기록(EHR)과 지역 네트워크를 활용한 환자 모집, 유지에 SMO의 전문성이 요구되고 있습니다. 분산 및 하이브리드 테스트로의 전환은 원격 모니터링, 디지털 리소스 및 데이터 정확도를 감독하는 SMO의 도입을 촉진합니다. 또한 아웃소싱 동향, 아시아태평양 및 라틴아메리카 등 신흥 시장의 성장, AI 및 전자 동의서(eConsent)와 같은 기술 통합이 더욱 확대되고 있습니다. 실세계 데이터(RWE) 및 시판 후 조사의 필요성이 높아지고 있는 점도 SMO를 확장성이 있는 고부가가치 네트워크로 변화시키고 있습니다.
시장 성장 억제요인
임상시험 지원기관(SMO) 시장은 직원 연수, 규제 준수, 기술 통합 등 높은 운영 경비로 인해 경쟁력 있는 가격 설정이 어려워지는 등 큰 제약에 직면하고 있습니다. 내부 지원 네트워크, SMO를 산하에 보유한 대기업 CRO, 대두하는 통합 연구기관(IRO)과의 격렬한 경쟁이 시장 점유율을 감소시키며 소규모 SMO는 틈새 분야에 대한 특화를 강요받고 있습니다. 환자의 모집과 유지는 여전히 지속되는 과제이며, SMO 이니셔티브가 있더라도 등록자가 부족하여 시험의 상당한 지연을 초래합니다. 궁극적으로 CRO와 스폰서 간의 계약 의존성과 신흥 시장의 경제적 변동으로 인해 SMO의 수익은 예측 불가능한 상태에 놓여 시장 확대에 지장을 초래하고 있습니다.
임상시험 지원기관(SMO) 시장 : 주요 인사이트
이 보고서는 임상시험 지원기관 시장의 현황을 상세하게 분석하여 업계 내 잠재적 성장 기회를 확인합니다. 주요 조사 결과는 다음과 같습니다.
1. 현재 세계 약 250명의 사업자들이 치료제품 및 의료기기 모두에 대해 임상시험 후원자를 위한 임상시험 지원 서비스를 제공하고 있습니다.
2. 시장은 세분화되어 있어 확립된 기업과 신규 진입기업이 모두 존재하며, 다양한 지역에서 폭넓은 치료 영역에 대해 시험 지원 서비스가 제공되고 있습니다.

3. 이 분야에 종사하는 기업은 서비스 포트폴리오의 확충과 진화하는 업계 기준과의 적합성을 도모하기 위해 지속적으로 능력을 확대하고 있습니다.
4. 지난 5년간 200건 이상의 계약이 체결되었으며, 보고된 제휴의 대부분은 스폰서 기업에 임상연구서비스 제공을 목적으로 하는 서비스 제휴 및 임상시험계약 체결에 초점이 맞춰져 있습니다.
5. 다수의 투자자들이 미래 수익성을 예측하고 임상시험 지원 서비스를 제공하는 기업에 29건에 걸쳐 총 10억 달러 상당의 자본을 투자하고 있습니다.

6. 2020년에는 전 세계적으로 약 10,000건의 임상시험이 등록되었으며 다양한 잠재적 개입법의 평가나 근거에 근거한 의료 및 헬스케어 솔루션의 개발이 진행되었습니다.
7. 임상시험수의 지속적인 증가에 따라 연구참가자에 대한 수요가 급증하고 있으며, 이에 따라 의약품 개발기업은 제3자 서비스 제공업체의 활용을 추진하고 있습니다.
8. 현재 임상시험 업무의 60% 이상이 외부 위탁되고 있는 상황을 근거로 향후 10년간 시장은 꾸준한 성장을 이룰 것으로 예측됩니다.

9. 예측되는 기회는 다양한 치료 영역에 분산될 것으로 예측됩니다. 또한 주요 지리적 지역에도 널리 분포할 가능성이 높습니다.
임상시험 지원기관(SMO) 시장
시장 규모 및 기회 분석은 다음 매개변수별로 세분화됩니다.
치료 영역
- 종양 질환
- 중추 신경계 질환
- 감염증
- 순환기 질환
- 기타
시험 단계
- 1상
- 2상
- 3상
- 4상
임상시험 구성 요소
- 시설 관리
- 현지 모니터링
- 프로젝트 관리
- 기타
개입 유형
- 치료
- 의료기기
- 수술 및 처치
주요 지역
- 북미
- 유럽
- 기타 지역
임상시험 지원기관(SMO) 시장 : 주요 부문
SMO 시장은 종양학적 적응증의 치료, 관리를 목적으로 한 임상시험이 주도할 전망입니다.
치료영역별로는 암질환, 중추신경계질환, 감염증, 호흡기질환, 심혈관질환, 내분비질환, 소화기질환, 근골격계질환, 면역질환, 피부질환 등으로 분류됩니다. SMO 시장은 2035년까지 시장 점유율의 38%를 차지할 전망이며, 종양학적 적응증의 치료, 관리를 목적으로 한 임상시험이 주도적 역할을 할 것으로 예측됩니다. 이는 세계적으로 종양학적 적응증을 표적으로 하는 약물의 잠재력을 조사하기 위해 현재 실시 중이거나 실시가 예정된 임상시험의 수가 증가하고 있기 때문입니다.
예측기간 중에는 2상 시험 연구용 SMO 시장이 주도적 지위를 차지할 전망
시험 단계별로 시장 전체는 1상, 2상, 3상, 4상으로 구분됩니다. 2상 시험 연구를 위한 SMO 시장은 올해에 약 40% 시장 점유율을 차지하며 대상 기간 동안 CAGR 12.1%를 보일 것으로 예측됩니다.
예측기간 동안 시설 관리 활동이 임상시험 지원기관 시장을 견인할 전망
임상시험 업무의 관점에서 전체 시장은 시설 관리, 데이터 관리, 품질 관리, 현지 제조, 규제 대응, 프로젝트 관리, 물류 등으로 구분됩니다. 시설 관리 활동(시설 선정, 계약, 결제, 시설 개시, 활성화, 시설 종료 포함)을 위한 SMO 시장은 올해도 약 30% 시장 점유율을 획득하고 예측 기간 동안 CAGR 9.8%로 성장할 것으로 전망됩니다.
치료제(의약품 및 생물학적 제제) 시장은 대상 기간 동안 CAGR 9.8%로 성장할 전망
치료제의 경우 전체 시장은 수술, 의료기기, 치료제로 구분됩니다. 치료제(의약품 및 생물학적 제제)를 위한 SMO 시장은 2035년까지 220억 달러 규모에 이르며 대상 기간 동안 CAGR 9.8%를 보일 것으로 예측됩니다.
북미는 임상시험 지원기관 시장의 성장을 견인할 전망
지역별로 시장 전체는 북미, 유럽, 아시아태평양, 라틴아메리카, 중동, 북아프리카(MENA) 및 기타 지역(RoW)으로 구분됩니다.
올해 북미는 임상시험 지원기관 시장 전체의 대부분의 점유율을 차지하고 있으며, 이 동향은 가까운 미래에도 변하지 않을 전망입니다. 그러나 아시아태평양은 예측 기간 동안 13.7%의 연평균 복합 성장률(CAGR)로 더 빠른 성장률을 보일 수 있습니다.
임상시험 지원기관 시장의 최근 동향
임상시험 지원기관 분야에서는 몇 가지 최근 동향을 볼 수 있습니다. 아래에 최근 동향의 일부를 소개합니다. 이러한 동향은 당사 시장 보고서 발표 후 발생하였으며, 분석에서 제시한 시장 전체의 동향을 뒷받침하는 것입니다.
- Psyence Biomed는 2b상 임상시험을 실시하기 위해 호주 임상시험 네트워크(ACTioN)와 전략적 제휴 계약을 체결했습니다.
- Flourish Research는 임상 연구 서비스의 추가 확대를 위해 Genstar Capital사로부터 전략적 투자를 유치했습니다.
- Neutra Corporation은 미국에 본사를 둔 연구시설 지원 기관인 Mercury Clinical Research를 인수했습니다. 또한 자산운용회사인 Blackstone은 도쿄에 거점을 두고 있는 임상시험 지원기관인 I'rom Group의 인수계획을 발표했습니다.
임상시험 지원기관 시장의 대표적인 진입기업
- FOMAT Medical Research
- Parexel
- Pharm-Olam
- Veristat
- WCCT Global
- Worldwide Clinical Trials
- CROMSOURCE
- Fidelis Research
- Scandinavian CRO
- TFS HealthScience
- Trialbee
- CMIC Group
- George Clinical
- Tigermed
- Veeda Clinical Research
목차
제1장 서문
제2장 주요 요약
제3장 소개
- 개요
- 임상시험 지원기관
- 임상시험에서의 임상시험 지원기관의 역할
- 임상시험 지원기관의 제공 서비스
- 원스톱 서비스의 장점
- 결론
제4장 경쟁 구도
- 개요
- 임상시험 지원기관 : 시장 정세
제5장 기업 경쟁력 분석
- 개요
- 전제 및 주요 파라미터
- 조사 방법
- 임상시험 지원기관 : 기업경쟁력 분석
제6장 기업 프로파일 : 북미의 임상시험 지원기관
- 개요
- FOMAT Medical Research
- Parexel
- Pharm-Olam
- Veristat
- WCCT Global
- Worldwide Clinical Trials
제7장 기업 프로파일 : 유럽의 임상시험 지원기관
- 개요
- CROMSOURCE
- Fidelis Research
- Scandinavian CRO
- TFS HealthScience
- Trialbee
제8장 기업 프로파일 : 아시아태평양의 임상시험 지원기관
- 개요
- CMIC Group
- George Clinical
- Tigermed
- Veeda Clinical Research
제9장 파트너십 및 협업
- 개요
- 파트너십 모델
- 임상시험 지원기관 : 파트너십 및 협업
제10장 자금 조달과 투자 분석
- 개요
- 자금 조달의 유형
- 임상시험 지원기관 : 자금조달 및 투자분석
제11장 임상시험 분석의 주요 인사이트
- 개요
- 범위와 조사 방법
- 임상시험 지원기관 : 임상시험의 주요 인사이트
제12장 임상시험 참가자 수요 분석
- 개요
- 조사 방법과 주요 전제
- 임상시험 참가자의 세계적 수요 : 등록환자 집단별 분석
제13장 시장 예측과 기회 분석
- 개요
- 예측 조사 방법과 주요 전제조건
- 세계의 임상시험 지원기관 시장(-2035년)
- 임상시험 지원기관 시장(2021년 및 2035년) : 치료영역별
- 임상시험 지원기관 시장(-2035년) : 시험 단계별
- 임상시험 지원기관 시장(-2035년) : 임상시험 구성요소별
- 임상시험 지원기관 시장(-2035년) : 개입 유형별
- 임상시험 지원기관 시장(2021년 및 2035년) : 지역별
제14장 결론
제15장 주요 인사이트
제16장 부록 1 : 표 형식 데이터
제17장 부록 2 : 기업 및 단체 일람
CSM 25.12.26SITE MANAGEMENT ORGANIZATION MARKET: OVERVIEW
As per Roots Analysis, the site management organization market is estimated to grow from USD 12.4 billion in the current year to USD 31. 6 billion by 2035, at a CAGR of 9.8% during the forecast period, till 2035.
SITE MANAGEMENT ORGANIZATIONS MARKET: GROWTH AND TRENDS
Clinical trials are a crucial step of the entire drug development process, allowing for the essential assessment of a drug candidate's safety and effectiveness. Research indicates that approximately 40% of the overall investment allocated for the advancement of drug candidates is spent on clinical trials, amounting to an annual cost of USD 78 billion. Nonetheless, conducting such trials frequently presents significant difficulties, including scientific and operational intricacies, challenges with recruitment and retention of appropriate patients, challenges associated with data management, and rigorous regulatory standards.
Additionally, due to the intrinsic complexity of the entire process and the participation of multiple stakeholders, these trials are susceptible to delays. Over 80% of clinical trials experience delays ranging from one to six months, whereas merely 10% of the studies finish on schedule. Consequently, innovators within the pharmaceutical sector are consistently working on enhancing methods for executing clinical trials and managing them effectively. Among other options, delegating different trial operations to a dedicated service provider, such as site management organizations (SMOs), has become a prominent choice for many developers. As the complexity increases and the number of registered clinical trials rises, the need for SMOs is expected to see consistent market growth throughout the forecast period.
Market Growth Drivers:
The site management organization (SMO) market is driven by increasing clinical trial volume and complexity, as registered studies require specialized site assistance for Phase II / III and precision medicine. Moreover, the increasing prevalence of chronic and rare diseases drives the need for SMOs' expertise in patient recruitment and retention via EHRs and community networks. The transition to decentralized and hybrid trials boosts SMO implementation for overseeing remote monitoring, digital resources, and data accuracy. Further, trends in outsourcing, growth of emerging markets in Asia-Pacific and LATAM, along with technology integration such as AI and eConsent propel further expansion. It is worth highlighting that the need for real-world evidence and post-marketing research is transforming SMOs into scalable, high-value networks.
Market Restraints:
The Site Management Organization (SMO) market faces considerable limitations due to high operational expenses, such as employee training, adherence to regulations, and technology integration which present competitive pricing challenges. Severe rivalry from internal site networks, major CROs with incorporated SMOs, and rising integrated research organizations (IROs) hampers market share and compels smaller SMOs to focus on niche sectors. Recruitment and retention of patients continue to pose challenges, leading to considerable trial delays from insufficient enrollment, even with SMO initiatives. Ultimately, reliance on CRO-sponsor agreements and economic fluctuations in emerging markets subject SMOs to revenue unpredictability, thus hindering their market expansion.
SITE MANAGEMENT ORGANIZATIONS MARKET: KEY INSIGHTS
The report delves into the current state of the site management organizations market and identifies potential growth opportunities within industry. Some key findings from the report include:
1. Presently, around 250 players across the globe claim to offer clinical trial site management services to trial sponsors, for both therapeutic products and medical devices.
2. The market is fragmented, featuring the presence of both established players and new entrants based in different geographies that claim to be capable of offering site management services, for wide range of therapeutic areas.
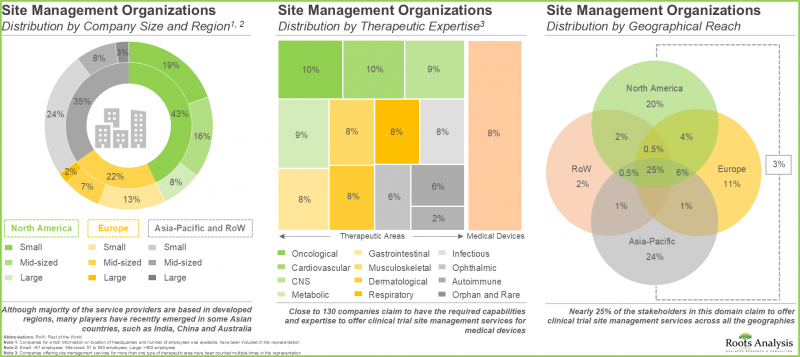
3. Companies involved in this domain are steadily expanding their capabilities in order to enhance their respective service portfolios and comply to evolving industry benchmarks.
4. Over 200 deals have been inked in the past five years; majority of the reported collaborations were focused on forming service alliances and clinical trial agreements to offer clinical research services to sponsors.
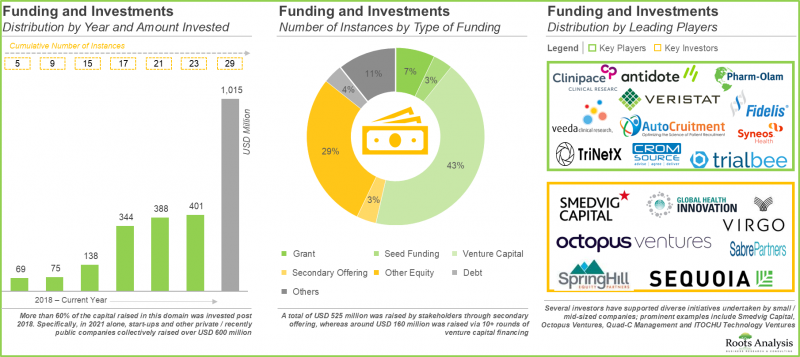
5. Foreseeing a lucrative future, a large number of investors have invested capital worth USD 1 billion, across 29 instances, in companies offering clinical trial site management services.
6. In 2020, around 10,000 trials were registered to evaluate various types of potential interventions and develop evidence-based medicine and health care solutions, worldwide.
7. There has been a surge in the demand for study participants owing to the continuous growth in number of clinical trials; this has prompted drug developers to leverage services to third party service providers.
8. With more than 60% of the clinical trial operations currently being outsourced, we expect the market to grow at a steady pace over the next decade.
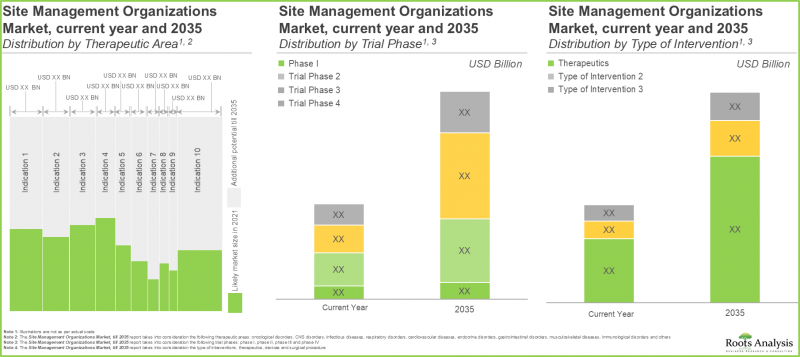
9. The anticipated opportunity is expected to be segregated across a variety of therapeutic areas; it is also likely to be well-distributed across key geographical regions.
Site Management Organization Market
The market sizing and opportunity analysis has been segmented across the following parameters:
Therapeutic Areas
- Oncological Disorders
- CNS Disorders
- Infectious Diseases
- Cardiovascular Diseases
- Others
Trial Phases
- Phase I
- Phase II
- Phase III
- Phase IV
Clinical Trial Components
- Site Management
- Onsite monitoring
- Project Management
- Others
Type of Interventions
- Therapeutics
- Devices
- Surgical Procedures
Key Geographies
- North America
- Europe
- Rest of the World
SITE MANAGEMENT ORGANIZATIONS MARKET: KEY SEGMENTS
SMOs Market is Likely to be Dominated by Trial Studies Intended for the Treatment / Management of Oncological Indications
In terms of therapeutic area, the market is segmented across oncological disorders, CNS disorders, infectious diseases, respiratory disorders, cardiovascular diseases, endocrine disorders, gastrointestinal disorders, musculoskeletal diseases, immunological disorders, dermatological disorders and others. The SMOs market is likely to be dominated by trial studies intended for the treatment / management of oncological indications, capturing 38% of the market share by 2035. This is owing to an increase in number of trials currently being / anticipated to be conducted to investigate the potential of drugs targeting oncological indications, worldwide.
SMOs market for Phase II Trial Studies is Likely to Dominate during the Forecast Period
In terms of the trial phase, the overall market is segmented across Phase I, Phase II, Phase III and Phase IV. The SMOs market for phase II trial studies is likely to capture nearly 40% of the market share in the current year, growing at a CAGR of 12.1%, during the given time period
Site Management Activities is Anticipated to Lead the Site Management Organization Market during the Forecast Period
In terms of clinical trial operations, the overall market is segmented across site management, data management, quality control, onsite manufacturing, regulatory affairs, project management, logistics and others. The SMOs market for site management activities (including site identification and selection, site contracting and payments, site initiation and activation and site close-out) is likely to capture around 30% of the market share in the current year, growing at a CAGR of 9.8%, during the given forecast period.
Market For Therapeutics (Drugs and Biologics) is Likely to Grow at a CAGR of 9.8%, During the Given Time Period
In terms of therapeutics, the overall market is segmented across surgical procedures, devices and therapeutics. the SMOs market for therapeutics (drugs and biologics) is likely to be worth USD 22 billion in 2035, growing at a CAGR of 9.8%, during the given time period.
North America is Likely to Propel the Growth of the Site Management Organization Market
In terms of geographical regions, the overall market is segmented across North America, Europe, Asia-Pacific, Latin America, MENA and RoW.
In the current year, North America captures the majority share of the overall clinical trial site management market and this trend is unlikely to change in the foreseen future. However, Asia-Pacific is likely to grow at a faster growth rate, with a CAGR of 13.7% during the forecast period.
Recent Developments in Site Management Organization Market:
Several recent developments have taken place in the field of site management organization. We have outlined some of these recent initiatives below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that have been outlined in our analysis.
- Psyence Biomed entered into a strategic agreement with Australian Clinical Trial Network (ACTioN) for its Phase IIb clinical trial.
- Flourish Research received strategic investment from Genstar Capital to further expand its clinical research services.
- Neutra Corporation acquired Mercury Clinical Research, a US based site management organization. Blackstone, an asset management company, also announced its plans to acquire Tokyo based SMO I'rom Group.
Primary Research Overview
The opinions and insights presented in the market report were also influenced by discussions held with senior stakeholders in the industry. The market research report features detailed transcripts of interviews held with the following individuals:
- Country Head - Clinical Operations, Mid-sized Company, India
- Medical Director and Operations Manager, Small Company, Argentina
- Project Manager, Small Company, India
Example Players in Site Management Organizations Market
- FOMAT Medical Research
- Parexel
- Pharm-Olam
- Veristat
- WCCT Global
- Worldwide Clinical Trials
- CROMSOURCE
- Fidelis Research
- Scandinavian CRO
- TFS HealthScience
- Trialbee
- CMIC Group
- George Clinical
- Tigermed
- Veeda Clinical Research
SITE MANAGEMENT ORGANIZATIONS MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the site management organizations market, focusing on key market segments, including [A] therapeutic area, [B] trial phases, [C] clinical trial components, [D] type of interventions, and [E] key geographical regions.
- Market Landscape: A detailed assessment of overall competitive landscape companies offering clinical trial management services to various organizations, including CROs, and pharmaceutical, biotechnology and medical devices companies based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] type of service offered, and [E] therapeutic expertise of service providers.
- Company Competitiveness Analysis: A comprehensive competitive analysis of service providers segregated into three peer groups based on location of their headquarters (North America, Europe, and Asia-Pacific and RoW), examining factors, such as [A] supplier strength [B] product strength and [C] application areas.
- Company Profiles: In-depth profiles of prominent players that offer various SMO services focusing on [A] year of establishment, [B] location of headquarters, [C] product portfolio, [D] recent developments and [E] an informed future outlook.
- Partnerships and Collaborations: An analysis of the partnerships that have been inked by stakeholders engaged in site management organization market, based on various parameters, such as [A] year of partnership, [B] type of partnership, [C] focus area and [D] most active players.
- Funding and Investment Analysis: A detailed analysis of various investments received by players engaged in site management organization market based on several relevant parameters, such as [A] year of investment, [B] number of funding instances, [C] amount invested, [D] type of funding (grant, seed, venture capital, secondary offering, other equity, debt and others) and [E] type of investor, [F] most active players, [G] most active investors and [H] geographical distribution (in terms of number of funding instances and amount invested).
- Clinical Trial Analysis: An in-depth analysis of completed, ongoing and planned clinical studies based on several relevant parameters, such as [A] trial registration year, [B] number of enrolled patients, [C] trial status, [D] trial phase, [E] type of sponsor and [F] geographical distribution of number of trials and enrolled patient population.
- Demand Analysis: An analysis of the annual demand for clinical study participants, taking into account the target patient population in ongoing and planned clinical trials, sponsored by both industry and non-industry players.
KEY QUESTIONS ANSWERED IN THIS REPORT
- What is a site-specific management organization and how does it operate?
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- Which region dominates the site management organizations market?
- What are the key trends observed in the site management organizations market?
- What factors are likely to influence the evolution of this market?
- What are the primary challenges faced by site management organizations?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Site Management Organizations
- 3.3. Role of Site Management Organizations in Clinical Trials
- 3.4. Services Offered by Site Management Organizations
- 3.5. Advantages of One-Stop-Shops
- 3.6. Concluding Remarks
4. COMPETITIVE LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Site Management Organizations: Overall Market Landscape
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Location of Headquarters
- 4.2.4. Analysis by Service(s) Offered
- 4.2.5. Analysis by Therapeutic Expertise
- 4.2.6. Analysis by Geographical Reach
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Assumptions and Key Parameters
- 5.3. Methodology
- 5.4. Site Management Organizations: Company Competitiveness Analysis
- 5.4.1. Competitiveness Analysis: Site Management Organizations in North America
- 5.4.1.1. Competitiveness Analysis: Small Companies in North America
- 5.4.1.2. Competitiveness Analysis: Mid-sized Companies in North America
- 5.4.1.3. Competitiveness Analysis: Large Companies in North America
- 5.4.2. Competitiveness Analysis: Site Management Organizations in Europe
- 5.4.2.1. Competitiveness Analysis: Small Companies in Europe
- 5.4.2.2. Competitiveness Analysis: Mid-sized Companies in Europe
- 5.4.2.3. Competitiveness Analysis: Large Companies in Europe
- 5.4.3. Competitiveness Analysis: Site Management Organizations in Asia-Pacific and Rest of the World
- 5.4.3.1. Competitiveness Analysis: Small Companies in Asia-Pacific and Rest of the World
- 5.4.3.2. Competitiveness Analysis: Mid-sized Companies in Asia-Pacific and Rest of the World
- 5.4.3.3. Competitiveness Analysis: Large Companies in Asia-Pacific and Rest of the World
- 5.4.1. Competitiveness Analysis: Site Management Organizations in North America
6. COMPANY PROFILES: SITE MANAGEMENT ORGANIZATIONS IN NORTH AMERICA
- 6.1. Chapter Overview
- 6.2. FOMAT Medical Research
- 6.2.1. Company Overview
- 6.2.2. Clinical Trial Site Management Services
- 6.2.3. Recent Developments and Future Outlook
- 6.3. Parexel
- 6.3.1. Company Overview
- 6.3.2. Clinical Trial Site Management Services
- 6.3.3. Recent Developments and Future Outlook
- 6.4. Pharm-Olam
- 6.4.1. Company Overview
- 6.4.2. Clinical Trial Site Management Services
- 6.4.3. Recent Developments and Future Outlook
- 6.5. Veristat
- 6.5.1. Company Overview
- 6.5.2. Clinical Trial Site Management Services
- 6.5.3. Recent Developments and Future Outlook
- 6.6. WCCT Global
- 6.6.1. Company Overview
- 6.6.2. Clinical Trial Site Management Services
- 6.6.3. Recent Developments and Future Outlook
- 6.7. Worldwide Clinical Trials
- 6.7.1. Company Overview
- 6.7.2. Clinical Trial Site Management Services
- 6.7.3. Recent Developments and Future Outlook
7. COMPANY PROFILES: SITE MANAGEMENT ORGANIZATIONS IN EUROPE
- 7.1. Chapter Overview
- 7.2. CROMSOURCE
- 7.2.1. Company Overview
- 7.2.2. Clinical Trial Site Management Services
- 7.2.3. Recent Developments and Future Outlook
- 7.3. Fidelis Research
- 7.3.1. Company Overview
- 7.3.2. Clinical Trial Site Management Services
- 7.3.3. Recent Developments and Future Outlook
- 7.4. Scandinavian CRO
- 7.4.1. Company Overview
- 7.4.2. Clinical Trial Site Management Services
- 7.4.3. Recent Developments and Future Outlook
- 7.5. TFS HealthScience
- 7.5.1. Company Overview
- 7.5.2. Clinical Trial Site Management Services
- 7.5.3. Recent Developments and Future Outlook
- 7.6. Trialbee
- 7.6.1. Company Overview
- 7.6.2. Clinical Trial Site Management Services
- 7.6.3. Recent Developments and Future Outlook
8. COMPANY PROFILES: SITE MANAGEMENT ORGANIZATIONS IN ASIA-PACIFIC
- 8.1. Chapter Overview
- 8.2. CMIC Group
- 8.2.1. Company Overview
- 8.2.2. Clinical Trial Site Management Services
- 8.2.3. Recent Developments and Future Outlook
- 8.3. George Clinical
- 8.3.1. Company Overview
- 8.3.2. Clinical Trial Site Management Service
- 8.3.3. Recent Developments and Future Outlook
- 8.4. Tigermed
- 8.4.1. Company Overview
- 8.4.2. Clinical Trial Site Management Services
- 8.4.3. Recent Developments and Future Outlook
- 8.5. Veeda Clinical Research
- 8.5.1. Company Overview
- 8.5.2. Clinical Trial Site Management Services
- 8.5.3. Recent Developments and Future Outlook
9. PARTNERSHIPS AND COLLABORATIONS
- 9.1. Chapter Overview
- 9.2. Partnership Models
- 9.3. Site Management Organizations: Partnerships and Collaborations
- 9.3.1. Analysis by Year of Partnership
- 9.3.2. Analysis by Type of Partnership
- 9.3.3. Analysis by Year and Type of Partnership
- 9.3.4. Analysis by Focus Area
- 9.3.5. Most Active Players: Analysis by Number of Partnerships
- 9.3.6. Geographical Analysis
- 9.3.6.1. Region-wise Distribution
- 9.3.6.2. Country-wise Distribution
10. FUNDING AND INVESTMENT ANALYSIS
- 10.1. Chapter Overview
- 10.2. Types of Funding
- 10.3. Site Management Organizations: Funding and Investment Analysis
- 10.3.1. Analysis by Year of Investment
- 10.3.2. Analysis by Amount Invested
- 10.3.3. Analysis by Type of Funding
- 10.3.4. Year-wise Analysis by Type of Funding and Amount Invested
- 10.3.5. Most Active Players: Analysis by Number of Funding Instances
- 10.3.6. Most Active Investors: Analysis by Number of Funding Instances
- 10.3.7. Analysis by Type of Investor
- 10.3.8. Analysis by Geography
11. KEY INSIGHTS FROM CLINICAL TRIAL ANALYSIS
- 11.1. Chapter Overview
- 11.2. Scope and Methodology
- 11.3. Site Management Organizations: Clinical Trial Key Insights
- 11.3.1. Analysis by Trial Registration Year
- 11.3.2. Analysis by Trial Registration Year and Enrolled Patient Population
- 11.3.3. Analysis by Trial Status
- 11.3.4. Analysis by Trial Registration Year and Trial Status
- 11.3.5. Analysis by Trial Phase
- 11.3.6. Analysis by Trial Phase and Enrolled Patient Population
- 11.3.7. Analysis by Trial Registration Year and Trial Phase (in terms of Number of Clinical Trials)
- 11.3.8. Analysis by Trial Registration Year and Trial Phase (in terms of Number of Enrolled Patient Population)
- 11.3.9. Analysis by Type of Sponsor / Collaborator
- 11.3.10. Geographical Analysis by Number of Clinical Trials
- 11.3.11. Geographical Analysis by Enrolled Patient Population
12. ANALYSIS OF DEMAND FOR CLINICAL TRIAL PARTICIPANTS
- 12.1. Chapter Overview
- 12.2. Methodology and Key Assumptions
- 12.3. Global Demand for Clinical Trial Participants: Analysis by Enrolled Patient Population
- 12.3.1. Analysis of Demand by Trial Phase
- 12.3.2. Analysis of Demand by Therapeutic Area
- 12.3.3. Geographical Demand by Enrolled Patient Population
13. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 13.1. Chapter Overview
- 13.2. Forecast Methodology and Key Assumptions
- 13.3. Global Site Management Organizations Market, Till 2035
- 13.4. Site Management Organizations Market, 2021 and 2035: Distribution by Therapeutic Area
- 13.5. Site Management Organizations Market, Till 2035: Distribution by Trial Phase
- 13.6. Site Management Organizations Market, Till 2035: Distribution by Clinical Trial Components
- 13.7. Site Management Organizations Market, Till 2035: Distribution by Type of Intervention
- 13.8. Site Management Organizations Market, 2021 and 2035: Distribution by Region
- 13.8.1. Site Management Organizations Market, 2021 and 2035: Distribution by Country
- 13.8.2. Site Management Organizations Market in North America, Till 2035
- 13.8.2.1. Site Management Organizations Market in the US, Till 2035
- 13.8.2.2. Site Management Organizations Market in Canada, Till 2035
- 13.8.2.3. Site Management Organizations Market in Rest of North America, Till 2035
- 13.8.3. Site Management Organizations Market in Europe, Till 2035
- 13.8.3.1. Site Management Organizations Market in the UK, Till 2035
- 13.8.3.2. Site Management Organizations Market in France, Till 2035
- 13.8.3.3. Site Management Organizations Market in Germany, Till 2035
- 13.8.3.4. Site Management Organizations Market in Spain, Till 2035
- 13.8.3.5. Site Management Organizations Market in Italy, Till 2035
- 13.8.3.6. Site Management Organizations Market in Rest of Europe, Till 2035
- 13.8.4. Site Management Organizations Market in Asia-Pacific, Till 2035
- 13.8.4.1. Site Management Organizations Market in China, Till 2035
- 13.8.4.2. Site Management Organizations Market in Korea, Till 2035
- 13.8.4.3. Site Management Organizations Market in India, Till 2035
- 13.8.4.4. Site Management Organizations Market in Australia, Till 2035
- 13.8.4.5. Site Management Organizations Market in Japan, Till 2035
- 13.8.4.6. Site Management Organizations Market in Israel, Till 2035
- 13.8.4.7. Site Management Organizations Market in Rest of Asia-Pacific, Till 2035
- 13.8.5. Site Management Organizations Market in Latin America, Till 2035
- 13.8.6. Site Management Organizations Market in MENA, Till 2035
- 13.8.7. Site Management Organizations Market in Rest of the World, Till 2035
14. CONCLUDING REMARKS
15. EXECUTIVE INSIGHTS
- 15.1. Chapter Overview
- 15.2. Company A
- 15.2.1. Company Snapshot
- 15.2.2. Interview Transcript: Country Head-Clinical Operations
- 15.3. Company B
- 15.3.1. Company Snapshot
- 15.3.2. Interview Transcript: Medical Director and Operations Manager
- 15.4. Company C
- 15.4.1. Company Snapshot
- 15.4.2. Interview Transcript: Project Manager












