
|
시장보고서
상품코드
1473268
<2024> 반고체전지 기술개발 현황 및 향후 전망<2024> Semi-Solid Battery Technology: Development and Future Prospects |
||||||
전고체전지에 대한 적용과 양산 계획이 주요 전지 메이저 및 자동차 OEM으로부터 발표가 되고 있으나, 대규모 양산에는 적어도 5-10년이 소요될 것으로 예측되는 가운데, 기존 LiB보다 안전성을 높이고, 긴 수명과 코스트 경제성을 갖춘 반고체전지에 대한 기대가 높아지고 있으며, 중국을 중심으로 많은 업체들이 속속 반고체전지 양산 및 적용 계획을 내놓고 있습니다.
반고체전지는 액체계에서 전고체전지로 전환되는 과도기 제품으로 간주되고 있으나, 전지 내부에 전해액 일부를 첨가하여 계면을 개선한 전지라고 할 수 있습니다. 반고체전지는 액체계에 비해 재료체계의 변화가 적고, 분리막과 액체전해질이 사용되며, 배터리 내부의 액체 전해질 함량을 줄임으로써 배터리의 비에너지용량과 안전성을 어느 정도 향상시킬 수 있으며, 대부분의 제조방법은 전통적인 리튬이온 배터리 기술과 장비 기술을 따릅니다.
반고체전지에서는 액체를 추가하면 복합재를 통한 이온 전달이 향상되고 전자 전달이 손상되지 않는 경우에 전극의 전기화학적 특성이 향상됩니다. 이를 위해서는 고체/액체 계면을 통한 이온 전달이 충분히 낮은 저항을 나타내야 합니다. 최근의 in-situ polymerization은 반고체전지의 원래 액체 성분을 응고시켜 액체 함량을 낮추는데 사용되었습니다.
반고체전지에서 가장 많은 형태의 하나인 유기-무기 복합 고체전해질은 유기 고체전해질과 무기 고체전해질의 장점을 결합한 것으로, 산화물 고체전해질과 고분자 전해질 복합재가 main으로 우수한 연성 및 기계적 강도, 우수한 가공성, 대량 생산에 적합한 충분한 이온 전도성을 나타냅니다.
CATL, WeLion, Qingtao Energy, Ganfeng Lithium, Guoxuan, Farasis, Tailan 등 대부분의 중국업체를 중심으로 진행중에 있으며, LGES, Factorial Energy, SES, ProLogium에서도 독자적 또는 자동차 OEM과 함께 개발하고 있습니다.
‘23년 4월 상하이 모터쇼에서 CATL은 응축형 배터리(凝聚??池-condensed Battery)를 발표하여 많은 관심을 받은 적이 있습니다. 이 배터리는 고출력 바이오닉 응축 전해질을 사용하고 마이크로 수준의 적응형 네트워크 구조를 구성하여 배터리의 동적 성능을 향상시키면서 리튬이온 수송 효율을 증가시킨 것으로서 셀 에너지밀도는 500Wh/kg에 달하며, 안정성 및 빠른 대량생산까지 가능한 것으로 시장의 관심을 받았습니다.
반고체전지의 적용은 이미 시작되고 있으며, WeLion의 360Wh/kg 에너지밀도의 반고체전지 150kWh를 탑재한 NIO ET7의 경우, 1145km를 주행하여 일반 배터리보다 더 먼 주행거리를 나타내었고, ’24. 4월부터 양산이 시작된다고 합니다.
SNE Research에 따르면, 글로벌 반고체전지 생산 CAPA는 ‘30년에 약 168GWh, ‘35년에 300GWh가 될 것으로 전망되며, 전체 전지에 대한 점유율을 보면, ‘30년 약 5.7%에서 ‘33년 6.55%로 peak를 보이다가 전고체전지가 본격적으로 도입되면서 ‘35년엔 다시 5.7%로 감소할 것으로 전망됩니다.
해당 리포트를 통해 리튬 메탈 산업 전반을 이해하고, insight가 들어간 SNE 리서치의 전망을 바라보며 리튬 원자재의 향후 변동을 대비할 수 있는 도움이 되기를 바랍니다.
본 보고서의 Strong Point는 다음과 같습니다.
- ① 반고체전지를 둘러싼 최근의 동향을 상세하게 수록
- ② 주요 전지 메이저들과 자동차 OEM들의 대처와 현황을 상세하게 수록
- ③ 반고체전지에 대한 시장 전망을 통해 전고체전지와의 경쟁 현황
- ④ 반고체전지의 지금까지의 개발이력과 개발 현황
- ⑤ 반고체전지 주요 특허 분석
목 차
1. 반고체전지 개발 동향
- 1.1. 전고체전지의 상용화 과제
- 1.1.1. 전고체전지 공정개발의 미성숙
- 1.2. 반고체전지의 산업체인에의 영향
- 1.2.1. 패키징-중간-후면 공정 변경
- 1.2.2. 고체전해질 성막 공정
- 1.2.3. 핵심 소재 시스템의 혁신
- 1.2.3.1. 고분자계 시스템
- 1.2.3.2. 산화물계 시스템
- 1.2.3.3. 황화물계 시스템
- 1.2.3.4. 코스트
- 1.2.3.5. 전해질
- 1.2.3.6. 음극재
- 1.2.3.7. 바인더
- 1.2.3.8. Pre-lithiation
- 1.2.3.9. 분리막
- 1.2.3.10. 건식전극공정
- 1.2.3.11. 반고체전지 산업화 현황
- 1.2.3.12. 겔형 고체전해질
- 1.3. 반고체전지 코스트 절감
- 1.3.1. 리튬금속 음극 적용
- 1.3.2. 규모 경제의 시동
- 1.4. 반고체전지의 주요 장점
2. 전고체, 준고체, 반고체, 하이브리드의 분류
- 2.1. 고체전해질 종류
- 2.2. 전해질 시스템 분류
- 2.3. 고체전해질 구분 및 장?단점
- 2.4. 하이브리드 전해질 종류
- 2.5-7. 하이브리드 전해질 특성
- 2.8. 하이브리드 복합전해질(IPC)
- 2.9-10. 유?무기 하이브리드 고체전해질
3. 반고체전지용 고분자/무기복합전해질
- 3.1. 개요
- 3.2. Introduction
- 3.2.1. 리튬 이온전도도
- 3.2.2. 전기화학적 안정성
- 3.2.3. 덴드라이트 억제
- 3.2.4. 접촉 안정성
- 3.3. 고분자/무기복합전해질 충진재(fillers)
- 3.3.1. 고분자/무기복합전해질의 구성 요소
- 3.3.2. 비활성 충진재(Inert Fillers)
- 3.3.2.1. 산화물 충진재
- 3.3.2.2. 강유전체 충진재
- 3.3.2.3. 다공성 충진재
- 3.3.2.4. 기타 충진재
- 3.3.3. 활성 충진재(Active Fillers)
- 3.3.3.1. Garnet형 충진재
- 3.3.3.2. NASICON형 충진재
- 3.3.3.3. Perovskite형 충진재
- 3.3.3.4. 황화물형 충진재
- 3.3.4. 고분자/무기고체전해질 합성
- 3.4. 결론 및 요약
4. 전고체/반고체전지 심층 분석
- 4.1. 전고체/반고체전지 장점(에너지밀도)
- 4.2. 전고체/반고체전지 장점(안전성)
- 4.3. 반고체전지 단점
- 4.4. 반고체전지 코스트, 원가절감 추세
- 4.5. 국가별 전고체/반고체전지 정책
- 4.6. 전고체/반고체전지 적용 시장
- 4.7. 반고체전지 적용 동향
- 4.8. 산화물 고체전해질 업체별 생산 CAPA
5. 반고체전지: EV의 최적 솔루션?
- 5.1. 반고체전지 vs. 기타 전지
- 5.2. 반고체전지 산업 체인
- 5.2.1. 양극 및 음극 소재
- 5.2.2. 고체전해질
- 5.2.3. EV 시장
- 5.2.3. 시장 분석(중국 시장)
6. 반고체 리튬전지 개발: Interlayer(중간층)도입
- 6.1. 연구 개요
- 6.2. Interlayer의 역할
- 6.3. Ag-C 복합재의 LLZTO와 통합
- 6.3.1. 추가층 도입
- 6.3.2. Ag의 접착력 향상
- 6.4. 리튬금속 셀의 특성 분석
- 6.4.1. 전기화학 셀
- 6.4.2. LLZTO의 전기화학적 성능
- 6.4.3. Li|Ag-C/Ag/LLZTO/IL|LCO 셀
- 6.5. 결과 및 논의
7. 고체전지용 하이브리드 계면(SLEI)
- 7.1. SLEI (Solid-Liquid Electrolyte Interphase)설명
- 7.2. LiB와 전고체전지의 차이
- 7.3. 하이브리드 고체/액체 전해질 시스템
- 7.4. SEI와 SLEI의 유사점과 차이
- 7.5. 산화물기반 하이브리드 시스템
- 7.6. 황화물기반 하이브리드 시스템
- 7.6.1. 순수 용매에 대한 연구
- 7.6.2. 액체 전해질에 대한 전기화학적 성능
8. 반고체 전해질 적용 고효율 리튬 금속전지(MOF도입)
- 8.1. 연구 개요
- 8.2. 준고체 전해질 제조
- 8.2.1. CuBTC MOF 호스트 물질
- 8.2.2. 준고체 전해질의 물리화학적 특성
- 8.2.3. 준고체 전해질과 전극과의 상용성
- 8.2.4. NCM-811//Li 파우치 셀의 전기화학적 성능
- 8.3. 결론
9. 반고체 전해질 적용 Li-S 전지
- 9.1. Li-S 전지 현황
- 9.2. 고체 Li-S 전지
- 9.2.1. 작동 원리
- 9.2.2. 충방전 프로파일
- 9.2.3. 이온전도 메커니즘
- 9.2.4. 기술적 Issues
- 9.3. 전해질 재료 설계 전략
- 9.4. 고분자 전해질 응용
- 9.4.1. 겔/준고체 고분자 전해질
- 9.4.2. 고체 고분자 전해질
- 9.5. 무기 고체전해질
- 9.5.1. 황화물 고체전해질
- 9.5.2. 산화물 고체전해질
- 9.6. 하이브리드 전해질
- 9.6.1. 유무기 복합전해질
- 9.6.2. 무기고체-액체전해질
- 9.7. 결론 및 전망
10. 반고체전지 산업 동향
- 10.1. 중국 반고체전지 현황
- 10.2. 중국 전고체전지 연구개발
- 10.3. 각 사의 투자 현황
- 10.4. 중국 전(반)고체전지 전망
- 10.5. 차세대 전지의 실용화
- 10.5.1. 현 LiB의 개선에 한계
- 10.5.2. 잇따른 반고체전지 개발
- 10.5.3. 반고체전지 인식 확산
- 10.5.4. 26년부터 전고체전지 생산
- 10.5.5. 3-7년 늦은 일본 기업
- 10.5.6. 반고체전지 글로벌 동향
- 10.5.7. 양극재/음극재 개발 동향
- 10.5.8. 배터리구조의 탈모듈화
- 10.5.9. 반고체전지 양산 돌입
11. 반고체전지 제조업체 및 운용 현황
- 11.1. LGES
- 11.2. SK On
- 11.3. WeLion
- 11.4. BAK
- 11.5. CATL
- 11.6. NIO
- 11.7. KAERI(한국)
- 11.8. ProLogium
- 11.9. SES
- 11.10. Factorial Energy
- 11.11. KIER(한국)
- 11.12. imec
- 11.13. Qingtao Energy
- 11.14. Ganfeng Lithium
- 11.15. Yiwei Lithium Energy (EVE)
- 11.16. Guoxuan Hi-Tech
- 11.17. Farasis Energy
- 11.18. SVOLT
- 11.19. SEMCORP
- 11.20. NGK
- 11.21. Kyocera
- 11.22. Talent New Energy
12. 반고체전지 시장 전망
- 12.1. 중국 반고체전지 산업현황
- 12.2. 반고체전지 산업 발전 역사
- 12.3. 중국 반고체전지 관련 정책
- 12.4. 중국 EV용 반고체전지 수요 전망
- 12.5. 중국 반고체전지 시장 침투율
- 12.6. 글로벌 반고체전지 시장 전망
13. 반고체전지 특허분석
- 13.1. 한국에너지기술연구원
- 13.2. Qingtao New Energy
- 13.3. LGES
- 13.4. Arkema
- 13.5. Global Graphene Group Inc.
- 13.6. WeLion New Energy
- 13.7. Ganfeng Lithium
- 13.8. Sion Power
- 13.9. Factorial Energy
- 13.10. StoreDot
- 13.11. SVOLT
Major battery manufacturers and automotive OEMs are planning to mass-produce all-solid-state batteries. However, large-scale production of these batteries is expected to take 5-10 years. In the meantime, there's increasing interest in semi-solid-state batteries due to their potential safety, longevity, and cost advantages over conventional lithium-ion batteries (LiBs). This anticipation is driving many companies, especially in China, to announce mass production plans for semi-solid-state batteries.
Semi-solid-state batteries serve as a transitional product between liquid-state and solid-state batteries. They incorporate a portion of electrolyte within the battery to enhance the interface. Compared to liquid-state batteries, semi-solid-state batteries necessitate minimal alterations to the material system, utilize separators and liquid electrolytes, and can decrease the volume of liquid electrolyte present. This reduction can enhance battery energy density and safety. Moreover, the manufacturing processes for semi-solid-state batteries closely align with traditional lithium-ion battery techniques and equipment.
In semi-solid-state batteries, the addition of a liquid can enhance ion transport through the composite material without compromising electron transport, leading to improved electrochemical performance of the electrodes. This requires a low-resistance ion transport through the solid/liquid interface. Recent in-situ polymerization techniques have been employed to reduce the liquid content by solidifying the original liquid components of the semi-solid-state battery.
Organic-inorganic composite solid electrolytes, one of the most prevalent forms in semi-solid-state batteries, combine the advantages of both organic and inorganic solid electrolytes. These composite materials, typically consisting of oxide solid electrolytes and polymer electrolytes, exhibit exceptional tensile strength and mechanical properties, excellent processability, and adequate ion conductivity, making them suitable for large-scale production.
The development of semi-solid-state batteries is primarily being driven by Chinese companies, including CATL, WeLion, Qingtao Energy, Ganfeng Lithium, Guoxuan, Farasis, and Tailan. Additionally, LGES, Factorial Energy, SES, and ProLogium are also independently or collaboratively developing this technology with automotive OEMs.
At the 2023 Shanghai Auto Show, CATL unveiled its highly anticipated Condensed Battery, grabbing industry attention. This groundbreaking technology utilizes a high-power bionic condensed electrolyte and features an adaptive micro-level network structure. These innovations enhance dynamic performance and lithium ion transport efficiency, resulting in an impressive cell energy density of 500 Wh/kg, surpassing conventional lithium-ion batteries. Moreover, CATL's innovation extends to stability and production capabilities, promising a reliable and scalable solution for electric vehicles in the future.
The application of solid-state batteries has already begun, and the NIO ET7 equipped with WeLion's 360Wh/kg semi-solid-state battery, boasting 150kWh, achieved a range of 1145km, surpassing conventional batteries. It's reported that mass production started in April 2024.
According to SNE Research, global solid-state battery production capacity is expected to reach approximately 168GWh by 2030 and 300GWh by 2035. In terms of overall battery market share, it is projected to peak at around 6.55% in 2033 from approximately 5.7% in 2030, before declining to 5.7% again by 2035 as solid-state batteries are widely adopted.
The strong points of this report are as follows.
- 1. Detailed coverage of recent trends surrounding semi-solid-state batteries
- 2. Detailed coverage of major battery manufacturers and automotive OEMs
- 3. Navigating the Competitive Landscape: Semi-Solid-State Batteries vs. Solid-State Batteries
- 4. The development history and current status of semi-solid-state batteries
- 5. Analysis of key patents related to semi-solid-state batteries
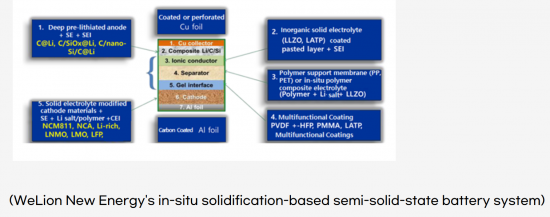
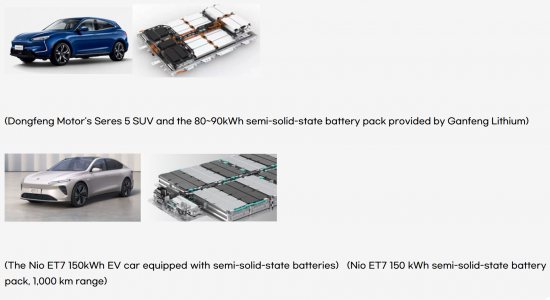

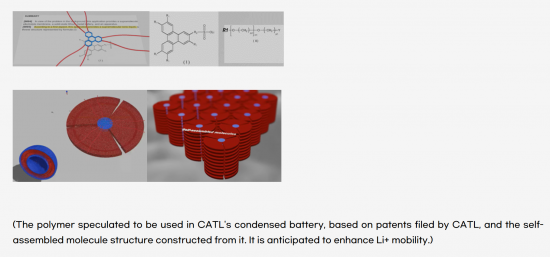
Table of Contents
1. Semi-Solid-State Battery Development Trends
- 1.1. Commercialization Challenges for All-Solid-State Batteries
- 1.1.1. Immaturity of All-Solid-State Battery Manufacturing
- 1.2. Semi-Solid-State Batteries: Impact on the Industrial Chain
- 1.2.1. Modifications to the Packaging Process: Intermediate and Rear Stages
- 1.2.2. Solid Electrolyte Film Formation Process
- 1.2.3. Innovation in Core Material Systems
- 1.2.3.1. Polymer Systems
- 1.2.3.2. Oxide Systems
- 1.2.3.3. Sulfide Systems
- 1.2.3.4. Costs
- 1.2.3.5. Electrolyte
- 1.2.3.6. Anode Material
- 1.2.3.7. Binders
- 1.2.3.8. Pre-lithiation
- 1.2.3.9. Separators
- 1.2.3.10. Dry Electrode Process (DBE)
- 1.2.3.11. Industrialization Status of Semi-Solid Batteries
- 1.2.3.12. Gel-Type Solid Electrolyte
- 1.3. Reducing the Cost of Semi-Solid-State Batteries
- 1.3.1. Using Lithium Metal Anodes
- 1.3.2. Need for Economies of Scale
- 1.4. Semi-Solid State Batteries: Key Advantages
2. Classification of Solid-State, Semi-Solid-State, and Hybrid Batteries
- 2.1. Types of Solid Electrolytes
- 2.2. Classification of Electrolyte Systems
- 2.3. Classification of Solid Electrolytes and Their Pros and Cons
- 2.4. Types of Hybrid Electrolyte
- 2.5~7. Properties of Hybrid Electrolyte
- 2.8. Hybrid Inorganic Polymer Composite Electrolyte (IPC)
- 2.9~10. Organic-Inorganic Hybrid Solid-State Electrolyte
3. Polymer/Inorganic Composite Electrolytes for Semi-Solid-State Batteries
- 3.1. Overview
- 3.2. Introduction
- 3.2.1. Lithium Ion Conductivity
- 3.2.2. Electrochemical Stability
- 3.2.3. Dendrite Suppression
- 3.2.4. Contact Stability
- 3.3. Polymer/Inorganic Composite Electrolyte Fillers
- 3.3.1. Components of Polymer/Inorganic Composite Electrolytes
- 3.3.2. Inert Fillers
- 3.3.2.1. Oxide Fillers
- 3.3.2.2. Ferroelectric Fillers
- 3.3.2.3. Porous fillers
- 3.3.2.4. Other Inert Fillers
- 3.3.3. Active Fillers
- 3.3.3.1. Garnet-Type Solid Electrolytes
- 3.3.3.2. NASICON-Type Solid Electrolytes
- 3.3.3.3. Perovskite-Type Solid Electrolytes
- 3.3.3.4. Sulfide-Type Solid Electrolyte
- 3.3.4. Synthesis of Polymeric/Inorganic Complex Electrolytes
- 3.4. Conclusion and Summary
4. In-Depth Analysis of All/Semi-Solid-State Batteries
- 4.1. All/Semi-Solid-State Battery Advantage (Energy Density)
- 4.2. All/Semi-Solid-State Battery Advantage (Safety)
- 4.3. Semi-Solid-State Batteries Disadvantage
- 4.4. Semi-Solid-State Battery Costs and Future Outlook
- 4.5. All/Semi-Solid-State Battery Policies by Country
- 4.6. All/Semi-Solid-State Battery Application Market
- 4.7. Semi-Solid-State Battery Application Trends
- 4.8. Oxide Solid Electrolyte Production Capacity by Company
5. Semi-Solid-State Batteries: The Optimal Solution for EVs?
- 5.1. Semi-Solid-State Batteries vs. Other Batteries
- 5.2. Semi-Solid-State Battery Industry Chains
- 5.2.1. Cathode/Anode Materials
- 5.2.2. Solid Electrolytes
- 5.2.3. EV Market
- 5.2.4. Market Analysis (Chinese Market)
6. Quasi-Solid Lithium Battery Development: Interlayer Application
- 6.1. Research Overview
- 6.2. The Role of the Interplayer
- 6.3. Integrating Ag-C Composites into LLZTO
- 6.3.1. Introducing Additional Layers
- 6.3.2. Enhancement of Ag Adhesion
- 6.4. Analysis of Lithium Metal Cell Characteristics
- 6.4.1. Electrochemical Cell
- 6.4.2. .Electrochemical Performance of LLZTO
- 6.4.3. Li-Ag-C/Ag/LLZTO/IL-LCO Cell
- 6.5. Conclusion and Discussion
7. Solid-State Battery Hybrid Interface (SLEI)
- 7.1. Definition of SLEI(Solid-Liquid Electrolyte Interphase)
- 7.2. Difference between LIBs and All-Solid-State Batteries
- 7.3. Hybrid Solid Electrolyte/Liquid Electrolyte(SE/LE) Systems
- 7.4. Similarities and differences between SLEI and SEI
- 7.5. Oxide-Based Hybrid System
- 7.6. Sulfide-Based Hybrid System
- 7.6.1. Research on Pristine Solvents
- 7.6.2. Electrochemical Performance for Liquid Electrolytes
8. High-Efficiency Li Metal Batteries with Quasi-Solid Electrolytes (Introduction of MOFs)
- 8.1. Research Overview
- 8.2. Fabrication of Quasi-Solid Electrolytes
- 8.2.1. CuBTC MOF as a Host Material
- 8.2.2. Physicochemical Properties of Quasi-Solid Electrolytes
- 8.2.3. Compatibility of Quasi-Solid Electrolytes with Electrodes
- 8.2.4. Electrochemical Performance of NCM-811//Li Pouch Cells
- 8.3. Conclusion
9. Li-S Batteries with Quasi-Solid Electrolytes
- 9.1. Li-S Battery Overveiw
- 9.2. Solid-State Li-S Batteries
- 9.2.1. Operating Principle
- 9.2.2. . Charge/Discharge Profile
- 9.2.3. Ion Conduction Mechanisms
- 9.2.4. Technical Issues
- 9.3. Electrolyte Material Design Strategies
- 9.4. Polymer Electrolyte Applications
- 9.4.1. Gel/Quasi-Solid Polymer Electrolyte
- 9.4.2. Solid-Polymer Electrolyte
- 9.5. Inorganic Solid Electrolyte
- 9.5.1. Sulfide Solid Electrolyte
- 9.5.2. Oxide Solid Electrolyte
- 9.6. Hybrid Electrolyte
- 9.6.1. Inorganic-Organic Composite Electrolyte
- 9.6.2. Inorganic Solid-Liquid Electrolyte
- 9.7. Conclusion and Outlook
10. Semi-Solid-State Battery Industry Trends
- 10.1. The State of Semi-Solid-State Batteries in China
- 10.2. .All-Solid-State Battery R&D in China
- 10.3. Each Company's Investment Status
- 10.4. Outlook on All(Semi)-Solid-State Batteries in China
- 10.5. Commercialization of Next-Generation Batteries
- 10.5.1. Limitations in Improving Current LiBs
- 10.5.2. Continued Development of Semi-Solid-State Batteries
- 10.5.3. Growing Recognition of Semi-Solid-State Batteries
- 10.5.4. Solid-State Battery Production from 2026
- 10.5.5. Japanese Companies Lagging Behind by 3-7 Years
- 10.5.6. Global Trends in Semi-Solid-State-Batteries
- 10.5.7. Trends in Cathode/Anode Material Development
- 10.5.8. Demodularization of the Battery Structure
- 10.5.9. Mass Production of Semi-Solid-State Batteries Initiated
11. Semi-Solid-State Battery Manufacturers and Operations
- 11.1. LGES
- 11.2. SK On
- 11.3. WeLion
- 11.4. BAK
- 11.5. CATL
- 11.6. NIO
- 11.7. KAERI(Korea)
- 11.8. ProLogium
- 11.9. SES
- 11.10. Factorial Energy
- 11.11. KIERKorea)
- 11.12. imec
- 11.13. Qingtao Energy
- 11.14. Ganfeng Lithium
- 11.15. Yiwei Lithium Energy (EVE)
- 11.16. Guoxuan Hi-Tech
- 11.17. Farasis Energy
- 11.18. SVOLT
- 11.19. SEMCORP
- 11.20. NGK
- 11.21. Kyocera
- 11.22. Talent New Energy
12. Semi-Solid State Battery Market Outlook
- 12.1. Current Status of Semi-Solid-State Battery Industry in China
- 12.2. History of Semi-Solid-State Battery Industry
- 12.3. Policies for China's Semi-Solid-State Battery
- 12.4. China's Semi-Solid-State Battery Demand Outlook for EVs
- 12.5. Market Penetration Rate of Semi-Solid-State Batteries in China
- 12.6. Global Semi-Solid-State Battery Market Outlook
13. Patent Analysis of Semi-Solid-State Batteries
- 13.1. Korea Institute of Energy Technology
- 13.2. Qingtao New Energy
- 13.3. LGES
- 13.4. Arkema
- 13.5. Global Graphene Group Inc.
- 13.6. WeLion New Energy
- 13.7. Ganfeng Lithium
- 13.8. Sion Power
- 13.9. Factorial Energy
- 13.10. StoreDot
- 13.11. SVOLT
References
(주말 및 공휴일 제외)


















