
|
연간정보 서비스
상품코드
1254616
치료용 mRNA 특허 추적조사Therapeutic mRNA Patent Monitor |
||||||
서비스의 주요 특징
분기별로 다음과 같은 최신 Excel 데이터베이스를 제공합니다:
- 새로운 특허 출원
- 새로 부여된 특허
- 기한 만료 또는 포기된 특허
- 지적재산권 양도(재양도, 라이선스), 지적재산권 공동 연구
- 특허 소송·이의제기
- 기술 구분, 용도, 치료 분야별로 분류된 특허 : mRNA 설계, 전달, 제조, mRNA 기반 백신, mRNA 기반 치료제, 감염증, 종양 질환, 기타 질환
- 업데이트된 온라인 데이터베이스에 대한 하이퍼링크(법적 지위, 문서 등)
분기별로 다음을 포함한 PDF 보고서를 제공합니다:
- 분기별 주요 실적과 수치
- 특허 현황을 포함하는 그래프와 코멘트
- 주요 IP 기업과 신규 진출 기업
IP 애널리스트로의 액세스(연간 100시간):
- mRNA 치료제 분야 동향, 분석, 특정 특허 기술, 기업 IP 포트폴리오에 관한 IP 애널리스트와의 Q&A 세션 및 논의
mRNA 치료제의 혜택을 활용 : 분기별 특허 추적조사 서비스
COVID-19 팬데믹에 대한 mRNA 백신의 성공은 mRNA 기반 치료 기술의 파괴적 측면을 조명하고 있습니다. COVID-19는 mRNA 기술 플랫폼의 많은 적응증 중 하나이며, 미생물 병원체나 암에 대한 넓은 범위에서의 새로운 백신으로서 높은 기대를 받고 있습니다. 또한 치료용 mRNA는 백신 접종뿐만 아니라 희귀 질환과 일반 질환(유전성 질환, 섬유성 질환, 자가면역 질환 등)의 치료용으로도 개발되고 있습니다. 그러나 mRNA를 일반적인 치료 수단으로서 확립하기 위해서는 일련의 과제가 남아 있습니다. 이러한 과제를 극복하기 위해 mRNA 수송을 최적화하는 접근법, 조직 트로피즘을 가진 운반체, mRNA 축적 등 다양한 신기술이 개발되고 있습니다. 또한 mRNA 백신과 치료제의 개발 경로는 mRNA의 면역원성, 조직 트로피즘, 전달, 약물동태 등 몇 가지 중요한 측면에서 다르기 때문에 향후 개발 방향은 치료 용도에 따라 달라질 수 있습니다.
이러한 급변하는 상황에서 다양한 기업의 지적재산 포지셔닝과 전략을 이해하는 것은 매우 중요합니다. 이러한 지식은 비지니스 위험과 기회를 감지하고, 새로운 연구 분야를 파악하고, 경쟁사의 전략을 이해하는데 도움이 됩니다.
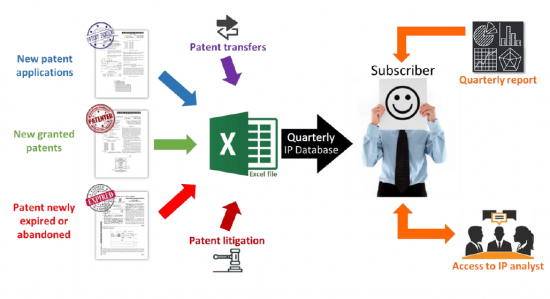
치료용 mRNA 특허 추적조사 서비스에서는 분기별로 업데이트되는 엑셀 파일을 통해 분기별 분석 보고서와 애널리스트와의 직접 대화의 혜택을 누릴 수 있습니다. 엑셀 파일에는 신규 특허 출원, 신규 취득 특허, 특허 만료/포기, 특허 양도(재양도, 라이선스), 특허 소송/이의제기가 포함됩니다. 특허는 mRNA 설계(자가증폭형과 고리형 RNA), 운반체, 제조, 저장 공정 등 기술 분야뿐만 아니라 용도 및 치료 분야별로도 분류되고 있습니다. 이 편리한 Excel 스프레드시트에는, 갱신된 온라인 데이터베이스에의 하이퍼 링크가 있어, 우선일, 특허 양수인, 특허 법적 지위, 특정 부문 등 다수의 기준으로 검색할 수 있습니다. 분기별 PDF 보고서는 지난 3개월간의 IP 동향을 주요 IP 기업 및 특허 기술에 대해 자세히 설명하고 있습니다. 애널리스트에게 직접 접속하여 분기별 보고서 결과, 동향, 분석, 특정 특허 기술 또는 치료용 mRNA 분야의 기업 특허 포트폴리오에 대한 온디맨드 Q&A 세션 및 공개 토론을 제공합니다.
특허 추적조사 서비스의 이점
경쟁사의 지적재산 활동이나 향후 의향을 파악
특허 추적조사 서비스를 이용하면 경쟁사의 현재 특허 활동, 지적재산 동태, 인수나 라이선스 등의 특허 이전, 특허 소송, 기술 개발, 연구개발 전략 등을 파악할 수 있습니다. 또한 자사 사업 분야 의 신규 진출 기업을 조기에 발견할 수 있습니다.
최신 기술 동향을 파악하고 기술 동향을 선점
최근의 특허 출원을 기록해 두면 해당 분야의 최신 기술 혁신을 추적할 수 있습니다. 특허 출원된 발명의 세부 사항을 알 수 있고, 기술 개발을 추적할 수 있습니다. 새로운 기술적인 해결책은 당신의 연구개발 활동을 자극하고 향상시킬 가능성이 있습니다.
비지니스에 악영향을 미칠 수 있는 지적재산권 등록 방지
독점적 권리가 부여되기 전에 특허 출원에 대한 정보를 얻을 수 있으며, 비즈니스에 악영향을 미칠 수 있는 지적재산권 등록을 방지하기 위해 제때 대응할 수 있습니다.
침해 행위에 대한 신속한 대응으로 법적 위험 감소
새로 발행된 특허를 모니터링하여 정기적으로 영업의 자유도를 평가할 수 있으며, 제품 및 공정이 특허의 대상이 아니며 타인의 유효한 지적재산권을 침해하지 않고 안전하게 제조, 판매 및 사용할 수 있는지 확인할 수 있습니다.
자유로운 기술 활용으로 연구개발 프로젝트의 위험을 저감
만료된 특허와 포기된 특허를 모두 추적하여 개발 시 안전하게 사용할 수 있는 퍼블릭 도메인에 진입한 발명을 식별할 수 있습니다.
경쟁사의 현재 지적재산 동향과 지적재산 전략을 이해
분기별로 지난 3개월간의 지적재산 동향을 제공하고, 주요 지적재산 기업, 신규 진출 기업, 주요 특허 기술에 대해 자세하게 설명합니다. 주요 특허 출원인과 그 발명, 블록 특허, 유망한 특허, 새로 만료되거나 포기된 주요 특허가 강조됩니다.
IP 애널리스트에 액세스
전화나 이메일로 애널리스트와 직접 소통하고, 온디맨드 Q&A 세션이나 논의를 통해 특정 특허 기술과 기업 IP 포트폴리오에 관한 구체적인 인풋을 얻을 수 있습니다(연간 100시간).

Key features of the service
Every quarter an up-to-date Excel database including:
- New patent applications
- Patent applications newly granted
- Expired or abandoned patents
- Transfer of IP rights (re-assignment, licensing) and IP collaborations.
- Patent litigations and oppositions
- Patents categorized by technological segmentation, application and therapeutic area: mRNA design, delivery, manufacturing, mRNA-based vaccines, mRNA-based therapeutics, infectious diseases, oncological disorders and other diseases.
- Hyperlinks to updated online database (legal status, documents etc.)
Every quarter a PDF report including:
- Key facts & figures of the quarter
- Graphs and comments covering the patent landscape evolutions
- A close look at the key IP players and newcomers
Access to an IP analyst for 100 hours per year:
- Q&A sessions and discussions with our IP analysts regarding trends, analyses, specific patented technologies, or companies' IP portfolios in the field of mRNA therapeutics.
Unlocking the benefits of mRNA therapeutics: A quarterly patent monitoring service
The success of mRNA vaccines as a response to the COVID-19 pandemic has shone a light on the disruptive aspect of mRNA-based therapeutic technology. COVID-19 is one of many indications for the mRNA technology platform, which holds high promise for new vaccines with a wide spectrum of microbial pathogens and cancers. Moreover, therapeutic mRNA is not limited to vaccination, but has also been developed to treat both rare and common diseases (genetic diseases, fibrotic diseases, autoimmune disorders, etc.). However, a series of challenges remain to be addressed before mRNA can be established as a general therapeutic modality. An array of new technologies is being developed to surmount these challenges, including approaches to optimize mRNA cargos, carriers with inherent tissue tropism, and mRNA storing, for example. The orientation of future development might also depend on the therapeutic application, as the development pathway for mRNA vaccines and therapeutics differs in several important respects, such as mRNA immunogenicity, tissue tropism, delivery, or pharmacokinetics.
In this fast-evolving context, it is crucial to understand the intellectual property position and strategy of the different players. Such knowledge can help detect business risks and opportunities, identify emerging research areas, and understand competitors' strategies.
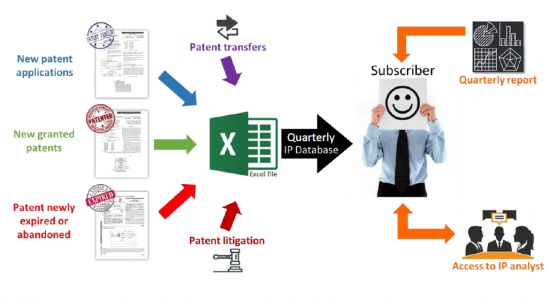
The mRNA Therapeutics Patent Monitoring Service allows you to take advantage of a quarterly-updated Excel file and benefit from both quarterly analysis reports and direct interaction with our analysts. The Excel file includes new patent applications, new granted patents, patents expired/abandoned, patent transfers (re-assignment, licensing), and patent litigation/opposition. The patents are categorized by technology segments, including mRNA design (self-amplifying and circular RNA), carriers, manufacturing, and storing process, but also by application and therapeutic area. This useful Excel spreadsheet with hyperlinks to updated online database allows for multi-criteria searches, including priority date, patent assignees, legal status of patents, and specific segments. The quarterly PDF reports provide the IP trends over the last three months, with a close look at key IP players and patented technologies. Direct access to our analysts offers you on-demand Q&A sessions and open discussions regarding the quarterly report results, trends, analyses, specific patented technologies, or companies' patent portfolios in the field of Therapeutic mRNA.
Benefits of the patent monitoring service
Keep an eye on your competitors' IP activities and their future intentions.
With the help of the patent monitoring service, you will be aware of your competitors' current patenting activities, their IP dynamics, patent transfers including acquisitions and licenses, patent litigation, technology development, and R&D strategies. You will also be able to detect newcomers early in your business area.
Keep track of the latest technology developments and stay ahead of technology trends.
By taking note of any recent patent filings, you can track the latest innovations in the field. You will get details on claimed inventions and you can follow technology developments. New technical solutions could inspire and improve your R&D activity.
Prevent registration of IP rights that may be harmful to your business.
You will obtain information on patent applications filed even before exclusive rights have been granted, and you can react in time to prevent registration of IP rights that may be harmful to your business.
React quickly to infringements and mitigate legal risks.
Monitoring newly-issued patents allows you to regularly assess your freedom-to-operate, ensuring that your products or processes are not covered by patents and thus can be manufactured, sold, or used safely without infringing valid IP rights owned by others.
Take advantage of free technologies and reduce R&D project risks.
By tracking both expired patents and abandoned patents, you will be able to identify inventions entering the public domain that you can use safely for your development.
Understand the current IP trends and IP strategy of competitors.
On a quarterly basis, the report will provide the IP trends over the last three months, with a close look at key IP players, newcomers, and key patented technologies. Main patent applicants and their inventions, blocking patents, promising patents, and key newly expired or abandoned patents will be highlighted.
Access an IP analyst.
Take advantage of direct interaction with our analysts by phone and/or email and get specific input for specific patented technologies and companies' IP portfolios through on-demand Q&A sessions and discussion (100 hours per year).

(주말 및 공휴일 제외)


















