
|
시장보고서
상품코드
1871286
RWE(Real World Evidence) 솔루션 시장 : 기회, 성장 요인, 업계 동향 분석 및 예측(2025-2034년)Real World Evidence Solutions Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
세계의 RWE(Real World Evidence) 솔루션 시장은 2024년 23억 달러로 평가되었으며, 2034년까지 연평균 복합 성장률(CAGR) 16.2%로 성장하여 102억 달러에 이를 것으로 예측됩니다.

시장 성장은 실시간 임상적 지식에 대한 수요 증가, 비용 효율적인 의약품 개발에 대한 수요 증가, 의료 분야에서의 빅데이터 분석의 활용 확대에 의해 견인되고 있습니다. RWE 솔루션은 의약 연구를 변화시키고 전자 건강 기록, 보험 데이터베이스 및 환자 등록 정보의 실제 세계 데이터를 사용하여 기업이 환자 결과, 치료 효과 및 안전성을 분석할 수 있도록 지원합니다. 의료 제공업체, 규제 당국, 지불 기관에 의한 근거에 근거한 의사결정에 대한 중시 증가가 시장의 성장을 더욱 가속화하고 있습니다. 또한 AI와 머신러닝의 진보로 RWE 플랫폼의 정확성과 예측 능력이 향상되어 임상시험의 설계와 시판후 조사의 신속화가 가능해지고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 기간 | 2025-2034년 |
| 시작 금액 | 23억 달러 |
| 예측 금액 | 102억 달러 |
| CAGR | 16.2% |
본 시장은 주로 구성요소별로 구분되어 있으며, 2024년 기준에서 서비스 분야가 58.4%의 점유율을 차지했습니다. 이러한 이점은 데이터 관리, 분석 및 규제 보고 업무가 전문 서비스 제공업체에 아웃소싱되는 경향이 커지고 있기 때문입니다. 서비스 분야는 바이오의약품 및 의료기기 제조업체가 RWE의 통합, 해석, 컴플라이언스 관리를 실시하는데 있어서 중요한 역할을 담당해, 시장 투입까지의 시간 단축과 조사 비용 절감에 공헌하고 있습니다.
최종 용도별로는 제약 및 의료기기 기업 부문이 2024년에 60.4%를 차지했습니다. 이것은 임상시험 환경을 넘어서는 약제의 효능 및 안전성의 실증 요구의 높아짐이 배경에 있습니다. 이 회사들은 환자 결과 개선 및 규제 준수 강화를 위해 적응 확대, 수명주기 관리 및 전략적 의사 결정에 RWE 솔루션을 활용합니다.
북미의 RWE 솔루션 시장은 2024년 43.2%의 점유율을 차지했으며, 이 지역의 첨단 의료 인프라, 지원 규제 프레임워크 및 디지털 건강 기술의 광범위한 채택을 통해 왔습니다. 미국은 주요 제약 기업의 존재, RWE 기반 임상 연구를 추진하는 강력한 정부 시책, 전자 건강 기록 및 청구 데이터베이스의 광범위한 이용으로 이 지역을 선도하고 있습니다. 정밀의료 및 환자 중심 케어에 대한 주력은 의료 생태계 전반에서 RWE 도입을 지속적으로 촉진하고 있습니다.
세계 RWE 솔루션 시장의 주요 기업으로는 IQVIA, Syneos Health, Oracle Corporation, Flatiron Health, ICON plc, Medidata Solutions 등이 있습니다. RWE 솔루션 시장의 기업은 전략적 제휴, 데이터 통합 능력, AI 구동형 분석 기술의 혁신을 통해 존재감을 강화하고 있습니다. 많은 기업들이 의료 제공업체나 지불기관과의 협업을 확대해 데이터 액세스의 향상과 실세계 연구의 품질 개선을 도모하고 있습니다. 주요 기업은 확장 가능한 클라우드 기반 플랫폼에 대한 투자를 추진하고 데이터 수집, 저장 및 해석의 효율성을 높이는 동시에 진화하는 데이터 프라이버시 규정을 준수합니다. 또한 합병과 인수를 활용하여 서비스 포트폴리오와 지리적 범위를 확대하고 있습니다.
자주 묻는 질문
목차
제1장 조사 방법과 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 미치는 영향요인
- 성장 촉진요인
- 의약품 개발 가속화와 비용 절감에 대한 주목의 고조
- 의약품 및 의료기기의 안전성 및 유효성에 관한 실시간 모니터링에 대한 수요 증가
- 정보에 근거한 상환 결정을 위한 RWE 솔루션 도입 증가
- 임상 의사 결정에 있어서의 데이터 분석 서비스의 채용 증가
- 업계의 잠재적 위험 및 과제
- 실세계 데이터의 통합 및 상호 운용성에서의 표준화 부족
- 숙련된 전문가의 부족
- 시장 기회
- 암 영역을 넘은 신흥 치료 영역의 확대
- 환자 생성 건강 데이터의 통합에 초점
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 북미
- 유럽
- 기술 상황
- 장래 시장 동향
- 갭 분석
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 소개
- 기업의 시장 점유율 분석
- 기업 매트릭스 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 전략 대시보드
- 주요 발전
- 인수합병
- 제휴 및 협력 관계
- 신제품 발매
- 확대 계획
제5장 시장 추계 및 예측 : 컴포넌트별, 2021-2034년
- 주요 동향
- 서비스
- 데이터 수집 및 통합
- 연구설계 및 실시
- 기댜 관찰 연구
- 후향 데이터베이스 연구
- 사이트 중심 연구
- 레지스트리 기반 연구
- 하이브리드 연구
- 규제 및 시장 접근 지원
- 증거 네트워크
- 기타 서비스
- 데이터 세트
- 이종 데이터 세트
- 임상 현장 데이터
- 청구 데이터 세트
- 약국 데이터 세트
- 환자 주도 데이터 세트
- 레지스트리 기반 데이터 세트
- 이종 데이터 세트
제6장 시장 추계 및 예측 : 용도별, 2021-2034년
- 주요 동향
- 의약품 개발 및 승인
- 종양학
- 순환기 질환
- 신경학
- 면역학
- 기타 치료 영역
- 의료기기 개발 및 승인
- 시판 후 조사
- 시장 접근과 상환 및 보험 적용에 관한 의사 결정
제7장 시장 추계 및 예측 : 수익 모델별, 2021-2034년
- 주요 동향
- 종량 과금(가치 기반 가격 설정)
- 구독
제8장 시장 추계 및 예측 : 도입 모델별, 2021-2034년
- 주요 동향
- On-Premise
- 클라우드 기반
제9장 시장 추계 및 예측 : 최종 용도별, 2021-2034년
- 주요 동향
- 제약 및 의료기기 제조업체
- 의료보험자
- 의료 제공업체
- 기타 최종 용도
제10장 시장 추계 및 예측 : 지역별, 2021-2034년
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제11장 기업 프로파일
- Aetion, Inc.
- Cytel Inc.
- Flatiron Health Inc.
- Fortrea Inc.
- ICON plc
- IBM Corporation
- IQVIA Holdings Inc.
- Medidata Solutions
- Merative
- Optum, Inc.
- Oracle Corporation
- Parexel International Corporation
- Syneos Health Inc.
- Tempus Labs Inc.
- Thermo Fisher Scientific Inc.
- TriNetX LLC.
The Global Real World Evidence Solutions Market was valued at USD 2.3 billion in 2024 and is estimated to grow at a CAGR of 16.2 % to reach USD 10.2 billion by 2034.
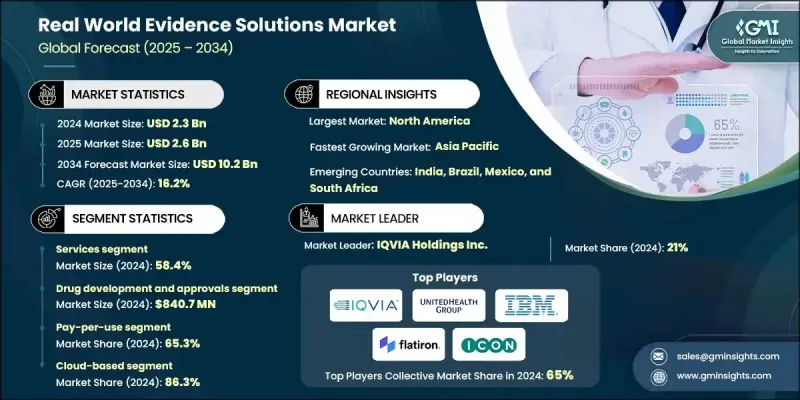
Market growth is driven by the rising need for real-time clinical insights, the growing demand for cost-effective drug development, and the expanding use of big data analytics in healthcare. RWE solutions are transforming pharmaceutical research, helping companies analyze patient outcomes, treatment efficacy, and safety using real-world data from electronic health records, insurance databases, and patient registries. The growing emphasis on evidence-based decision-making by healthcare providers, regulators, and payers is further fueling market growth. Moreover, advancements in AI and machine learning are enhancing the accuracy and predictive capabilities of RWE platforms, enabling faster clinical trial design and post-market surveillance.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2.3 Billion |
| Forecast Value | $10.2 Billion |
| CAGR | 16.2% |
The market is primarily segmented by component, with the services segment held 58.4% share in 2024. This dominance is attributed to the increasing outsourcing of data management, analytics, and regulatory reporting to specialized service providers. The services segment plays a crucial role in assisting biopharma and medical device firms in real-world data integration, interpretation, and compliance management, reducing time-to-market and research costs.
In terms of end-use, the pharmaceutical and medical devices companies segment held 60.4% in 2024, driven by the growing need to demonstrate drug effectiveness and safety beyond clinical trial environments. These companies are leveraging RWE solutions for label expansion, lifecycle management, and strategic decision-making to improve patient outcomes and regulatory compliance.
North America Real World Evidence Solutions Market held 43.2% share in 2024, owing to the region's advanced healthcare infrastructure, supportive regulatory frameworks, and widespread adoption of digital health technologies. The U.S. leads the region due to the presence of major pharmaceutical companies, strong government initiatives promoting RWE-based clinical research, and extensive use of electronic health records and claims databases. The region's focus on precision medicine and patient-centric care continues to drive RWE adoption across healthcare ecosystems.
Key players operating in the Global Real World Evidence Solutions Market include IQVIA, Syneos Health, Oracle Corporation, Flatiron Health, ICON plc, and Medidata Solutions. Companies in the Real World Evidence Solutions Market are strengthening their presence through strategic partnerships, data integration capabilities, and AI-driven analytics innovations. Many are expanding collaborations with healthcare providers and payers to enhance data accessibility and improve real-world study quality. Key players are also investing in scalable cloud-based platforms to streamline data capture, storage, and interpretation while ensuring compliance with evolving data privacy regulations. Additionally, mergers and acquisitions are being leveraged to expand service portfolios and geographic reach.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Component trends
- 2.2.3 Application trends
- 2.2.4 Revenue model trends
- 2.2.5 Deployment trends
- 2.2.6 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing focus towards accelerating drug development and cost reduction
- 3.2.1.2 Growing demand for real-time safety and efficacy monitoring of drugs and medical devices
- 3.2.1.3 Increasing adoption of RWE Solutions for informed reimbursement decision-making
- 3.2.1.4 Increasing adoption of data analytics services in clinical decision making.
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Lack of standardization in integration and interoperability of real-world data
- 3.2.2.2 Shortage of skilled professionals
- 3.2.3 Market opportunities
- 3.2.3.1 Emerging therapeutic areas expansion beyond oncology
- 3.2.3.2 Focus on patient-generated health data integration
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.5 Technology landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
- 4.7 Key developments
- 4.7.1 Mergers and acquisitions
- 4.7.2 Partnerships and collaborations
- 4.7.3 New product launches
- 4.7.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Component, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Services
- 5.2.1 Data collection and integration
- 5.2.2 Study design and execution
- 5.2.2.1 Prospective observational studies
- 5.2.2.2 Retrospective database studies
- 5.2.2.3 Site-centric studies
- 5.2.2.4 Registry-based studies
- 5.2.2.5 Hybrid studies
- 5.2.3 Regulatory and market access support
- 5.2.4 Evidence network
- 5.2.5 Other services
- 5.3 Data sets
- 5.3.1 Disparate data sets
- 5.3.1.1 Clinical settings data
- 5.3.1.2 Claims data sets
- 5.3.1.3 Pharmacy data sets
- 5.3.1.4 Patient powered data sets
- 5.3.1.5 Registry-based data sets
- 5.3.1 Disparate data sets
Chapter 6 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Drug development and approval
- 6.2.1 Oncology
- 6.2.2 Cardiovascular disease
- 6.2.3 Neurology
- 6.2.4 Immunology
- 6.2.5 Other therapeutic areas
- 6.3 Medical device development and approvals
- 6.4 Post-market surveillance
- 6.5 Market access and reimbursement/coverage decision-making
Chapter 7 Market Estimates and Forecast, By Revenue Model, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pay-per-use (value-based pricing)
- 7.3 Subscription
Chapter 8 Market Estimates and Forecast, By Deployment Model, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 On-premise
- 8.3 Cloud-based
Chapter 9 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Pharmaceutical and medical device companies
- 9.3 Healthcare payers
- 9.4 Healthcare providers
- 9.5 Other End use
Chapter 10 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 10.1 Key trends
- 10.2 North America
- 1.1.1 U.S.
- 1.1.2 Canada
- 10.3 Europe
- 1.1.3 Germany
- 1.1.4 UK
- 1.1.5 France
- 1.1.6 Spain
- 1.1.7 Italy
- 1.1.8 Netherlands
- 10.4 Asia Pacific
- 1.1.9 China
- 1.1.10 Japan
- 1.1.11 India
- 1.1.12 Australia
- 1.1.13 South Korea
- 10.5 Latin America
- 1.1.14 Brazil
- 1.1.15 Mexico
- 1.1.16 Argentina
- 10.6 Middle East and Africa
- 1.1.17 South Africa
- 1.1.18 Saudi Arabia
- 1.1.19 UAE
Chapter 11 Company Profiles
- 11.1 Aetion, Inc.
- 11.2 Cytel Inc.
- 11.3 Flatiron Health Inc.
- 11.4 Fortrea Inc.
- 11.5 ICON plc
- 11.6 IBM Corporation
- 11.7 IQVIA Holdings Inc.
- 11.8 Medidata Solutions
- 11.9 Merative
- 11.10 Optum, Inc.
- 11.11 Oracle Corporation
- 11.12 Parexel International Corporation
- 11.13 Syneos Health Inc.
- 11.14 Tempus Labs Inc.
- 11.15 Thermo Fisher Scientific Inc.
- 11.16 TriNetX LLC.



















