
|
시장보고서
상품코드
1666711
eClinical 솔루션 시장 기회, 성장 촉진요인, 산업 동향 분석, 예측(2025-2034년)eClinical Solutions Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
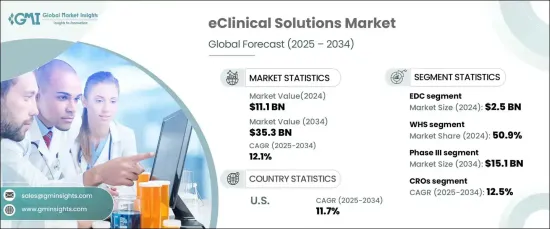
세계의 eClinical 솔루션 시장은 2024년에 111억 달러에 달하며, 2025-2034년에 CAGR 12.1%로 성장할 것으로 예측됩니다.
이러한 급성장의 배경에는 디지털 기술을 활용하여 데이터 수집을 효율화하고 환자 참여를 향상시키는 분산형 임상시험의 채택이 증가하고 있는 것이 주요 요인으로 꼽힙니다. 웨어러블 기기, 모바일 헬스 용도, 전자건강기록의 사용이 증가하면서 실시간 모니터링과 데이터 정확도가 향상되고 있습니다.
규제기관 또한 임상시험의 효율성을 최적화하고 환자 안전 기준을 유지하기 위해 디지털 툴을 옹호하고 있습니다. 또한 맞춤형 의료 및 만성질환 치료의 발전에 힘입어 전 세계에서 임상시험의 양이 증가함에 따라 첨단 e임상솔루션에 대한 필요성이 증가하고 있습니다. 이러한 플랫폼은 환자 모집, 컴플라이언스 모니터링, 실시간 데이터 분석과 같은 복잡한 프로세스를 간소화하여 점점 더 복잡해지는 대규모 임상시험을 관리하는 데 필수적인 역할을 하고 있습니다.
| 시장 범위 | |
|---|---|
| 시작연도 | 2024년 |
| 예측연도 | 2025-2034년 |
| 시작 금액 | 111억 달러 |
| 예상 금액 | 353억 달러 |
| CAGR | 12.1% |
e임상솔루션에는 임상시험 관리를 간소화하기 위해 설계된 첨단 소프트웨어와 디지털 플랫폼이 포함되어 있습니다. 이러한 툴은 전자 데이터 수집(EDC) 시스템, 임상시험 관리 시스템(CTMS), 전자 환자 보고 결과(ePRO) 등 여러 소스의 데이터를 통합하여 정확성을 높이고 규제 준수를 보장하며, EDC 분야는 데이터 처리 강화, 수작업 오류 최소화, 실시간 모니터링 가속화 등의 기능을 통해 2024년까지 25억 달러 규모 시장을 창출할 것으로 예상됩니다. 실시간 모니터링 가속화 등의 기능으로 2024년 25억 달러 규모 시장을 창출하며 시장을 선도하고 있습니다. 임상시험이 복잡해짐에 따라 이러한 시스템에 대한 수요는 지속적으로 증가하고 있습니다.
2024년 시장 점유율은 웹호스트형 솔루션이 50.9%로 가장 큰 비중을 차지했습니다. 확장성, 경제성, 도입 용이성으로 인해 분산형 임상시험에 이상적입니다. 이러한 솔루션은 원격 액세스, 다른 디지털 툴와의 원활한 통합, 강력한 보안 기능을 통해 그 보급을 촉진하고 있습니다. 실시간 협업과 데이터 공유를 촉진하는 기능을 통해 시장에서의 입지를 더욱 공고히 하고 있습니다.
임상 3상 시험 분야는 2024년에 시장을 장악하고 2034년에는 151억 달러에 달할 것으로 예상됩니다. 이 단계에서는 치료의 안전성과 유효성을 평가하기 위한 대규모 시험이 진행되므로 효율적인 데이터 관리와 모니터링이 필요하며, e클리니컬 툴은 실시간 데이터 수집을 가능하게 하고 규제 요건을 준수할 수 있도록 함으로써 이러한 프로세스를 강화합니다. 강화합니다.
임상시험 수탁기관(CRO)은 예측 기간 중 12.5%의 연평균 복합 성장률(CAGR)로 성장할 것으로 예상되며, CRO의 전문성과 효율성으로 인해 임상연구를 CRO에 위탁하는 경향이 증가하고 있는 것이 시장 성장의 원동력이 되고 있습니다. 이들 조직은 e임상솔루션을 활용하여 복잡한 다국가의 임상시험을 관리하고, 업무를 간소화하며, 실시간 데이터 분석을 실현하기 위해 e임상솔루션을 활용하고 있습니다.
미국은 2024년 북미 시장을 장악하고 CAGR 11.7%로 선두를 유지할 것으로 예상됩니다. 이는 미국의 탄탄한 제약 및 생명공학 산업과 기술 혁신 및 임상시험의 분산화에 중점을 두고 있기 때문입니다. 주요 연구기관과 규제기관의 존재는 첨단 e-임상기술의 채택을 더욱 촉진하고 있습니다.
목차
제1장 조사 방법과 조사 범위
제2장 개요
제3장 업계 인사이트
- 업계 에코시스템 분석
- 업계에 대한 영향요인
- 성장 촉진요인
- 임상시험에 대한 정부 보조금의 증가
- CRO에 대한 임상시험 아웃소싱의 수요 증가
- 임상시험에서 소프트웨어 솔루션의 채택 증가
- 세계에서 연구개발비의 증가
- 업계의 잠재적 리스크·과제
- 높은 도입 비용
- 신흥 국가에서 eClinical 솔루션의 낮은 보급률과 인지도
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 소프트웨어 상황
- Porter의 산업 분석
- PESTEL 분석
제4장 경쟁 구도
- 서론
- 기업 시장 매트릭스 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 전략 대시보드
제5장 시장 추산·예측 : 솔루션별, 2021-2034년
- 주요 동향
- 무작위화와 임상시험 공급 관리
- 임상 데이터 관리 시스템(CDMS)
- 임상시험 관리 시스템(CTMS)
- 전자 임상결과평가(eCOA)
- 전자 임상시험 마스터 파일(eTMF)
- 전자 데이터 수집(EDC)
- 기타 솔루션
제6장 시장 추산·예측 : 딜리버리 모드별, 2021-2034년
- 주요 동향
- 기업용(온프레미스) 라이선싱 솔루션
- 클라우드 기반(SAAS) 솔루션(CBS)
- 웹 호스트형(온디맨드) 솔루션(WHS)
제7장 시장 추산·예측 : 임상시험 단계별, 2021-2034년
- 주요 동향
- 단계 I
- 단계 II
- 단계 III
- 단계 IV
제8장 시장 추산·예측 : 최종 용도별, 2021-2034년
- 주요 동향
- CRO(임상시험 수탁기관 기관)
- 의료기기 기업
- 제약/바이오테크놀러지 기업
- 병원·클리닉
- 기타 최종 용도
제9장 시장 추산·예측 : 지역별, 2021-2034년
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 폴란드
- 스위스
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 인도네시아
- 태국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 아르헨티나
- 칠레
- 중동 및 아프리카
- 남아프리카공화국
- 사우디아라비아
- 아랍에미리트
- 이집트
제10장 기업 개요
- Acralyon
- Anju Software(OmniComm System)
- Bio-optronics
- Clario
- Dassault Systemes(Medidata)
- Datatrak
- Ennov
- IBM
- Medsharing
- Multihealth Group(Clinfile)
- OpenClinica
- Oracle Corporation
- Parexel International
- Signant Health(CRF Health)
- Veeva Systems
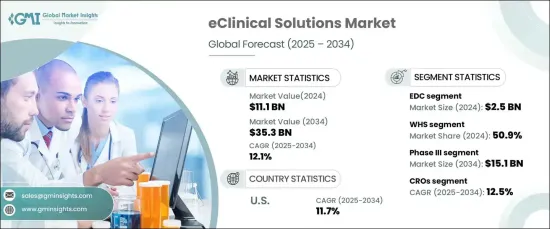
The Global EClinical Solutions Market, valued at USD 11.1 billion in 2024, is forecasted to grow at a CAGR of 12.1% from 2025 to 2034. This rapid growth is driven by the increasing adoption of decentralized clinical trials, which utilize digital technologies to streamline data collection and improve patient engagement. The rising use of wearable devices, mobile health applications, and electronic health records is enhancing real-time monitoring and data accuracy.
Regulatory bodies are also advocating for digital tools to optimize trial efficiency and maintain patient safety standards. Additionally, the escalating volume of clinical trials worldwide, fueled by advancements in personalized medicine and treatments for chronic conditions, is amplifying the need for advanced eClinical solutions. These platforms simplify complex processes such as patient recruitment, compliance monitoring, and real-time data analytics, making them essential in managing the growing intricacies of large-scale trials.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $11.1 Billion |
| Forecast Value | $35.3 Billion |
| CAGR | 12.1% |
eClinical solutions encompass advanced software and digital platforms designed to streamline clinical trial management. These tools integrate data from multiple sources, including electronic data capture (EDC) systems, clinical trial management systems (CTMS), and electronic patient-reported outcomes (ePRO), ensuring improved accuracy and regulatory compliance. The EDC segment led the market in 2024, generating USD 2.5 billion, due to its ability to enhance data processing, minimize manual errors, and accelerate real-time monitoring. As trials become more complex, the demand for such systems continues to rise.
Web-hosted solutions accounted for the largest market share of 50.9% in 2024. Their scalability, affordability, and ease of deployment make them ideal for decentralized trials. These solutions enable remote access, seamless integration with other digital tools, and robust security features, driving their widespread adoption. Their ability to facilitate real-time collaboration and data sharing has further solidified their market position.
The phase III clinical trial segment dominated the market in 2024, projected to reach USD 15.1 billion by 2034. This phase involves large-scale testing to evaluate treatment safety and efficacy, necessitating efficient data management and monitoring. eClinical tools enhance these processes by enabling real-time data capture and ensuring compliance with regulatory requirements.
Contract research organizations (CROs) are set to grow at a 12.5% CAGR over the forecast period. The rising trend of outsourcing clinical research to CROs, driven by their expertise and efficiency, is a key market driver. These organizations utilize eClinical solutions to manage complex multinational trials, streamline operations, and ensure real-time data analysis.
The US dominated the North American market in 2024 and is expected to maintain its lead with a CAGR of 11.7%. This is attributed to the country's robust pharmaceutical and biotechnology industries, as well as its emphasis on technological innovation and decentralized trials. The presence of major research institutions and regulatory bodies further supports the adoption of advanced eClinical technologies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rise in government funding grants for clinical trials
- 3.2.1.2 Growing demand for outsourcing clinical trials to CROs
- 3.2.1.3 Increasing adoption of software solutions in clinical trials
- 3.2.1.4 Increasing R&D expenditure across the globe
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High implantation cost
- 3.2.2.2 Low adoption and lack of awareness pertaining to eClinical solutions in developing countries
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Software landscape
- 3.6 Porter’s analysis
- 3.7 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Solution, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Randomization and trial supply management
- 5.3 Clinical data management system (CDMS)
- 5.4 Clinical trial management system (CTMS)
- 5.5 Electronic clinical outcome assessment (eCOA)
- 5.6 Electronic trial master file (eTMF)
- 5.7 Electronic data capture (EDC)
- 5.8 Other solutions
Chapter 6 Market Estimates and Forecast, By Delivery Mode, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Licensed enterprise (on-premise) solutions
- 6.3 Cloud-based (SAAS) solution (CBS)
- 6.4 Web-hosted (on-demand) solution (WHS)
Chapter 7 Market Estimates and Forecast, By Clinical Trial Phase, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Phase I
- 7.3 Phase II
- 7.4 Phase III
- 7.5 Phase IV
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Contract research organizations (CROs)
- 8.3 Medical device companies
- 8.4 Pharma/biotech companies
- 8.5 Hospitals and clinics
- 8.6 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Poland
- 9.3.7 Switzerland
- 9.3.8 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.4.6 Indonesia
- 9.4.7 Thailand
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.5.4 Argentina
- 9.5.5 Chile
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
- 9.6.4 Egypt
Chapter 10 Company Profiles
- 10.1 Acralyon
- 10.2 Anju Software (OmniComm System)
- 10.3 Bio-optronics
- 10.4 Clario
- 10.5 Dassault Systemes (Medidata)
- 10.6 Datatrak
- 10.7 Ennov
- 10.8 IBM
- 10.9 Medsharing
- 10.10 Multihealth Group (Clinfile)
- 10.11 OpenClinica
- 10.12 Oracle Corporation
- 10.13 Parexel International
- 10.14 Signant Health (CRF Health)
- 10.15 Veeva Systems



















