
|
시장보고서
상품코드
1708182
종양학 동반진단 시장 기회, 성장 촉진요인, 산업 동향 분석 및 예측(2025-2034년)Oncology Companion Diagnostic Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
종양학 동반진단 세계 시장은 2024년 46억 달러에 달했고, 2025-2034년 10.8%의 연평균 복합 성장률(CAGR)을 보일 것으로 예측됩니다.
암 동반진단은 암 치료에서 해당 약물 및 생물학적 제제를 안전하고 효과적으로 사용할 수 있는 중요한 통찰력을 제공하는 중요한 도구입니다. 이러한 진단은 환자의 종양에서 특정 유전자 마커, 돌연변이, 바이오마커를 찾아내어 의료진이 환자가 표적 치료에 반응할 가능성이 있는지 여부를 판단할 수 있도록 돕습니다. 정밀의료의 도입이 진행됨에 따라 기존 치료법에서 환자별 종양 특성 및 유전적 프로파일에 따라 치료법을 조정하는 개인 맞춤형 접근법으로 초점이 이동하고 있습니다. 이러한 추세는 암 동반진단 의약품에 대한 수요를 촉진하는 주요 동인입니다.
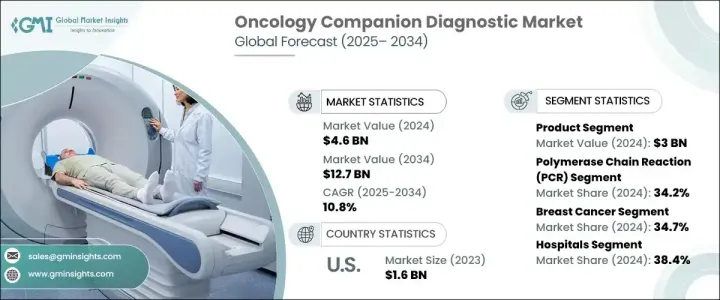
시장은 제품, 기술, 질병 유형, 최종 용도별로 구분됩니다. 서비스별로 시장은 제품 및 서비스로 나뉩니다. 기기, 소모품, 소프트웨어를 포함한 제품 부문은 2024년 30억 달러의 매출을 기록했고, 예측 기간 동안 연평균 10.6%의 성장률을 보일 것으로 예측됩니다. 중합효소연쇄반응(PCR) 시스템 및 차세대 염기서열분석기(NGS)와 같은 장비는 유전자 변이 검출에 있어 높은 민감도와 정확도를 제공합니다. 시약, 분석 키트, 프라이머, 프로브 등의 소모품은 정확한 결과를 보장합니다. 인공지능(AI) 및 머신러닝(ML) 등의 고급 알고리즘은 예측 정확도를 향상시키고 합리적인 데이터 분석을 가능하게 합니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 46억 달러 |
| 예상 금액 | 127억 달러 |
| CAGR | 10.8% |
기술별로 PCR, NGS, 면역조직화학(IHC), in situ hybridization(ISH)/fluorescence in situ hybridization(FISH), 기타 기술로 분류되며, PCR은 2024년 34.2%의 시장 점유율을 차지했고, 2034년에는 45억 달러에 달할 것으로 예측됩니다. PCR 시스템은 미량의 DNA 또는 RNA를 검출할 때 높은 민감도와 효율성을 제공하며, 몇 시간 내에 빠른 결과를 제공합니다. 디지털 PCR(dPCR) 및 실시간 PCR(qPCR)과 같은 PCR 기술의 발전으로 한 번의 검사로 여러 암 바이오마커를 동시에 검출할 수 있게 되어 비용 절감과 시간 단축이 가능해졌습니다.
질병 유형별로는 유방암, 비소세포폐암, 대장암, 백혈병, 흑색종, 전립선암, 기타로 분류됩니다. 유방암 분야는 주로 유병률 증가로 인해 2024년 34.7%의 점유율로 시장을 장악헀습니다. 최종 사용자 세분화에는 병원, 진단 실험실, 학술 및 연구 기관, 기타 최종 사용자가 포함됩니다. 병원은 2024년 시장 점유율의 38.4%를 차지했는데, 이는 입원 및 외래 환자 모두 대량의 암 환자를 관리할 수 있는 능력이 있기 때문입니다. 동반진단약은 병원이 맞춤 치료를 관리하고 부작용을 줄이며 생존율을 향상시킬 수 있는 능력을 부여합니다.
미국은 암 발병률 증가와 미국 식품의약국(FDA)이 동반진단 의약품에 대한 엄격한 기준을 마련하고 있어 여전히 주요 시장으로 자리 잡고 있습니다. 이러한 규제는 보다 안전하고 효과적인 진단 키트 및 진단 장비의 개발을 촉진하여 이 지역의 암 동반진단 시장의 전반적인 성장을 가속하고 있습니다.
목차
제1장 조사 방법과 조사 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 대한 영향요인
- 성장 촉진요인
- 업계의 잠재적 리스크&과제
- 성장 가능성 분석
- 규제 상황
- 기술적 전망
- 향후 시장 동향
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 서론
- 기업 점유율 분석
- 기업 매트릭스 분석
- 주요 시장 기업 - 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 전략 대시보드
제5장 시장 추산 및 예측 : 제공 제품별, 2021년-2034년
- 주요 동향
- 제품
- 기기
- 소모품
- 소프트웨어
- 서비스
제6장 시장 추산 및 예측 : 기술별, 2021년-2034년
- 주요 동향
- 중합효소 연쇄반응(PCR)
- 차세대 시퀸싱(NGS)
- 면역조직화학(IHC)
- In situ hybridization (ISH)/fluorescence in situ hybridization (FISH)
- 기타 기술
제7장 시장 추산 및 예측 : 질환 유형별, 2021년-2034년
- 주요 동향
- 유방암
- 비소세포 폐암
- 대장암
- 백혈병
- 흑색종
- 전립선암
- 기타 질환 유형
제8장 시장 추산 및 예측 : 최종 용도별, 2021년-2034년
- 주요 동향
- 병원
- 진단 실험실
- 학술기관 및 연구기관
- 기타 최종 용도
제9장 시장 추산 및 예측 : 지역별, 2021년-2034년
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카공화국
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 개요
- Abbott Laboratories
- Agilent Technologies
- BioMérieux
- Biocartis
- Bio-Rad Laboratories
- EntroGen
- F. Hoffmann-La Roche
- Foundation Medicine
- Guardant Health
- Illumina
- Leica Biosystems
- Myriad Genetics
- QIAGEN
- Sysmex Corporation
- Thermo Fisher Scientific
The Global Oncology Companion Diagnostic Market reached USD 4.6 billion in 2024 and is expected to grow at a CAGR of 10.8% from 2025 to 2034. Oncology companion diagnostics are essential tools that provide critical insights into the safe and effective use of corresponding drugs or biological products in cancer treatment. These diagnostics help identify specific genetic markers, mutations, and biomarkers in a patient's tumor, enabling healthcare providers to determine whether a patient is likely to respond to a targeted therapy. The increasing adoption of precision medicine has shifted the focus from traditional treatment methods to personalized approaches that tailor therapies based on a patient's unique tumor characteristics and genetic profile. This trend is a key driver fueling the demand for oncology companion diagnostics.
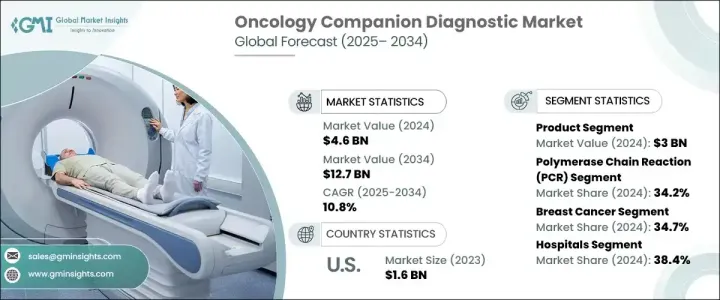
The market is segmented by offering, technology, disease type, and end-use. By offering, the market is divided into products and services. The product segment, which includes instruments, consumables, and software, generated USD 3 billion in revenue in 2024 and is projected to grow at a CAGR of 10.6% during the forecast period. Instruments such as polymerase chain reaction (PCR) systems and next-generation sequencers (NGS) offer high sensitivity and accuracy in detecting genetic mutations. Consumables, including reagents, assay kits, primers, and probes, ensure accurate results. Software solutions play a pivotal role in analyzing and interpreting complex genomic and clinical data, with advanced algorithms such as artificial intelligence (AI) and machine learning (ML) improving predictive accuracy and enabling streamlined data interpretation.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4.6 Billion |
| Forecast Value | $12.7 Billion |
| CAGR | 10.8% |
The market is also categorized by technology into PCR, NGS, immunohistochemistry (IHC), in situ hybridization (ISH)/fluorescence in situ hybridization (FISH), and other technologies. PCR held a 34.2% market share in 2024 and is expected to reach USD 4.5 billion by 2034. PCR systems provide high sensitivity and efficiency in detecting minimal amounts of DNA or RNA, delivering quick results within hours, which is essential for timely clinical decision-making. Advancements in PCR technologies, such as digital PCR (dPCR) and real-time PCR (qPCR), allow for the simultaneous detection of multiple cancer biomarkers in a single test, reducing costs and saving time.
By disease type, the market is divided into breast cancer, non-small cell lung cancer, colorectal cancer, leukemia, melanoma, prostate cancer, and others. The breast cancer segment dominated the market with a 34.7% share in 2024, primarily due to the rising prevalence of the disease. End use segmentation includes hospitals, diagnostic laboratories, academic and research institutions, and other end users. Hospitals accounted for 38.4% of the market share in 2024, attributed to their capacity to manage high volumes of cancer patients, both inpatient and outpatient. Companion diagnostics empower hospitals to manage personalized treatments, reducing side effects and improving survival rates.
The United States remains a key market due to the increasing incidence of cancer and the stringent standards established by the U.S. Food and Drug Administration (FDA) for companion diagnostics. These regulations have encouraged the development of safer, more effective diagnostic kits and instruments, enhancing the overall growth of the oncology companion diagnostic market in the region.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of cancer
- 3.2.1.2 Advancements in genomic technologies
- 3.2.1.3 Rising demand for targeted therapies
- 3.2.1.4 Increased investment in research and development
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High development costs
- 3.2.2.2 Stringent regulatory requirements
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Offering, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Product
- 5.2.1 Instrument
- 5.2.2 Consumables
- 5.2.3 Software
- 5.3 Services
Chapter 6 Market Estimates and Forecast, By Technology, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Polymerase chain reaction (PCR)
- 6.3 Next-generation sequencing (NGS)
- 6.4 Immunohistochemistry (IHC)
- 6.5 In situ hybridization (ISH)/fluorescence in situ hybridization (FISH)
- 6.6 Other technologies
Chapter 7 Market Estimates and Forecast, By Disease Type, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Breast cancer
- 7.3 Non-small cell lung cancer
- 7.4 Colorectal cancer
- 7.5 Leukemia
- 7.6 Melanoma
- 7.7 Prostate cancer
- 7.8 Other disease types
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Diagnostic laboratories
- 8.4 Academic and research institutions
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott Laboratories
- 10.2 Agilent Technologies
- 10.3 BioMérieux
- 10.4 Biocartis
- 10.5 Bio-Rad Laboratories
- 10.6 EntroGen
- 10.7 F. Hoffmann-La Roche
- 10.8 Foundation Medicine
- 10.9 Guardant Health
- 10.10 Illumina
- 10.11 Leica Biosystems
- 10.12 Myriad Genetics
- 10.13 QIAGEN
- 10.14 Sysmex Corporation
- 10.15 Thermo Fisher Scientific












