
|
시장보고서
상품코드
1721607
체외진단(IVD) 시장 기회, 성장 촉진요인, 산업 동향 분석 및 예측(2025-2034년)In-vitro Diagnostics Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
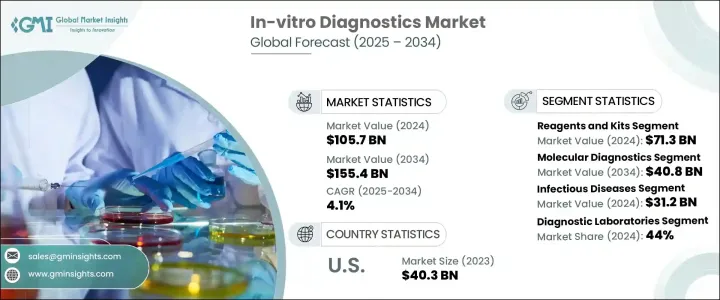
세계의 체외진단(IVD) 시장은 2024년 1,057억 달러로 평가되었고, CAGR 4.1%를 나타내 2034년에는 1,554억 달러에 달할 것으로 예측됩니다. 이 도구는 질병의 감지, 진단 및 관리에 중요한 역할을 하며, 보다 빠르고 정확한 임상 판단을 가능하게 합니다.

IVD 솔루션은 병원과 임상 실험실 뿐만 아니라, 재택치료나 포인트 오브 케어에서의 채용도 늘고 있습니다. 헬스 플랫폼의 채용은 결과의 생성, 해석, 제공 방법을 변화시키고, 개별화된 실시간 케어에 새로운 길을 열고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 1,057억 달러 |
| 예측 금액 | 1,554억 달러 |
| CAGR | 4.1% |
시약 및 키트 분야는 2024년에 713억 달러를 창출했고, 2025년부터 2034년에 걸쳐 CAGR 4.4%를 나타낼 것으로 예측됩니다. 이 성장은 정확한 조기 진단에 대한 요구가 높아지고 맞춤형 치료 계획에 대한 수요가 증가함에 따라 시약 및 키트에는 생화학 시약, 항체, 분석 키트, 프로브, 프라이머 등 다양한 진단 검사에 필수적인 폭넓은 제품이 포함되어 있습니다.
분자진단 분야는 2024년에 25.5% 시장 점유율을 차지했으며, 2034년에는 408억 달러에 이르는 등 대폭적인 성장이 전망되고 있습니다. 이 도구는 전염병, 유전성 질환 및 암을 감지하기 위한 선호되는 옵션입니다. 결과가 나오기까지 며칠이 걸리는 전통적인 검사와는 달리, 분자진단은 몇 시간에서 몇 분 이내에 실용적인 지식을 얻을 수 있기 때문에 보다 신속한 치료 방침의 결정과 환자의 결과를 개선할 수 있습니다.
북미의 체외진단(IVD) 시장은 2024년 451억 달러를 창출했고 강력한 건강관리 인프라, 만성질환의 고부담, 첨단기술의 조기 도입으로 지배적인 지위를 유지하고 있습니다.
세계의 체외진단(IVD) 시장 주요 기업은 Abbott Laboratories, ACON Laboratories, Agilent Technologies, Becton, Dickinson and Company, Bio-Rad Laboratories, Dragerwerk, F. Hoffmann-La Roche, Danaher Corporation, Medtronic, Meridian Bioscience, Nova Biomedical, PerkinElmer, Siemens Healthineers, Sysmex Corporation 등이 있습니다.
목차
제1장 조사 방법과 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 미치는 영향요인
- 성장 촉진요인
- 세계적으로 감염과 만성 질환의 만연
- 맞춤형 의료 보급과 수요 증가
- 신흥 국가의 포인트 오브 케어 검사 도입 증가
- 고도의 진단 기기를 갖춘 병리학 연구실이나 서비스 급증
- 업계의 잠재적 위험 및 과제
- 진단 서비스의 고비용
- 불리한 상환 시나리오
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 기술적 상황
- 향후 시장 동향
- 갭 분석
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 서론
- 기업의 시장 점유율 분석
- 기업 매트릭스 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 전략 대시보드
제5장 시장 추계·예측 : 제품별(2021-2034년)
- 주요 동향
- 시약 및 키트
- 기기
제6장 시장 추계·예측 : 검사 유형별(2021-2034년)
- 주요 동향
- 임상 화학
- 면역 분석/면역 화학
- 분자 진단
- 혈액학
- 소변 검사
- 기타 검사 유형
제7장 시장 추계·예측 : 용도별(2021-2034년)
- 주요 동향
- 종양학
- 감염성 질환
- 당뇨병
- 심장학
- 신장학
- 자가면역질환
- 약물 검사/약물 유전체학
- 기타 용도
제8장 시장 추계·예측 : 최종 용도별(2021-2034년)
- 주요 동향
- 병원
- 진단 실험실
- 학술 및 연구 기관
- 기타 용도
제9장 시장 추계·예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 아시아태평양
- 중국
- 인도
- 일본
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 사우디아라비아
- 남아프리카
- 아랍에미리트(UAE)
제10장 기업 프로파일
- Abbott Laboratories
- ACON Laboratories
- Agilent Technologies
- Becton, Dickinson and Company
- Biomerieux
- Bio-Rad Laboratories
- Dragerwerk
- F. Hoffmann-La Roche
- Danaher Corporation
- Medtronic
- Meridian Bioscience
- Nova Biomedical
- PerkinElmer
- Siemens Healthineers
- Sysmex Corporation
The Global In-Vitro Diagnostics Market was valued at USD 105.7 billion in 2024 and is estimated to grow at a CAGR of 4.1% to reach USD 155.4 billion by 2034. In-vitro diagnostics (IVD) includes a wide range of medical devices, reagents, and systems used to analyze biological samples such as blood, urine, and tissues outside the human body. These tools play a vital role in detecting, diagnosing, and managing diseases, enabling faster and more accurate clinical decisions. As healthcare systems around the world continue to shift toward preventive care and precision medicine, the demand for advanced diagnostic tools is rising steadily.
IVD solutions are increasingly being adopted not only in hospitals and clinical laboratories but also in home care and point-of-care settings. The growing burden of chronic and infectious diseases, rising awareness about early disease detection, and the emergence of novel diagnostic technologies are collectively driving the expansion of this market. Moreover, the adoption of artificial intelligence and digital health platforms in diagnostics is transforming the way results are generated, interpreted, and delivered, creating new avenues for personalized and real-time care. Supportive regulatory policies, favorable reimbursement scenarios, and a strong push for healthcare digitization across developed and emerging economies further accelerate the industry's growth trajectory.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $105.7 Billion |
| Forecast Value | $155.4 Billion |
| CAGR | 4.1% |
The reagents and kits segment generated USD 71.3 billion in 2024 and is expected to grow at a CAGR of 4.4% from 2025 to 2034. This growth is largely driven by the increasing need for precise and early-stage diagnosis, as well as the rising demand for customized treatment plans. Reagents and kits cover a broad spectrum of products, including biochemical reagents, antibodies, assay kits, probes, and primers, all of which are essential for running a variety of diagnostic tests. With the advancement of molecular diagnostics, these products are now capable of delivering highly accurate and faster results compared to traditional testing methods, making them indispensable in modern diagnostic labs.
The molecular diagnostics segment held a 25.5% market share in 2024 and is expected to grow significantly, reaching USD 40.8 billion by 2034. This segment benefits from ongoing innovations that enhance test accuracy, turnaround time, and ease of use. Molecular diagnostic tools are now the preferred option for detecting infectious diseases, genetic disorders, and cancer, thanks to their ability to identify pathogens and genetic mutations at a molecular level. Unlike conventional tests that often require days to return results, molecular diagnostics can produce actionable insights within hours or even minutes, enabling faster treatment decisions and better patient outcomes.
The North America In-Vitro Diagnostics Market generated USD 45.1 billion in 2024, maintaining a dominant position due to strong healthcare infrastructure, a high burden of chronic illnesses, and early adoption of advanced technologies. Despite a stringent regulatory landscape, the region continues to support the approval and commercialization of cutting-edge diagnostic solutions.
Key players in the Global In-Vitro Diagnostics Market include Abbott Laboratories, ACON Laboratories, Agilent Technologies, Becton, Dickinson and Company, Bio-Rad Laboratories, Dragerwerk, F. Hoffmann-La Roche, Danaher Corporation, Medtronic, Meridian Bioscience, Nova Biomedical, PerkinElmer, Siemens Healthineers, and Sysmex Corporation. These companies focus on research and development, strategic partnerships, and global expansion to meet the rising demand for advanced diagnostic technologies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of infectious and chronic diseases worldwide
- 3.2.1.2 Growing adoption and rising demand for personalized medicine
- 3.2.1.3 Increasing adoption of point-of-care testing in developing countries
- 3.2.1.4 Surging number of pathology labs and services equipped with advanced diagnostics machine
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of diagnostic services
- 3.2.2.2 Unfavorable reimbursement scenario
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Reagents and kits
- 5.3 Instruments
Chapter 6 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Clinical chemistry
- 6.3 Immunoassay/immunochemistry
- 6.4 Molecular diagnostics
- 6.5 Hematology
- 6.6 Urinalysis
- 6.7 Other test types
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Oncology
- 7.3 Infectious diseases
- 7.4 Diabetes
- 7.5 Cardiology
- 7.6 Nephrology
- 7.7 Autoimmune diseases
- 7.8 Drug testing/pharmacogenomics
- 7.9 Other applications
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Diagnostic laboratories
- 8.4 Academic and research institutes
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott Laboratories
- 10.2 ACON Laboratories
- 10.3 Agilent Technologies
- 10.4 Becton, Dickinson and Company
- 10.5 Biomerieux
- 10.6 Bio-Rad Laboratories
- 10.7 Dragerwerk
- 10.8 F. Hoffmann-La Roche
- 10.9 Danaher Corporation
- 10.10 Medtronic
- 10.11 Meridian Bioscience
- 10.12 Nova Biomedical
- 10.13 PerkinElmer
- 10.14 Siemens Healthineers
- 10.15 Sysmex Corporation



















