
|
시장보고서
상품코드
1883940
IVD 랩웨어 시장 : 제품별(플라스틱 및 유리), 유형별, 용도별, 최종 사용자별 - 예측(-2030년)IVD Labware Market by Product (Plastic, Glass ), Type, Application, End User - Global Forecast to 2030 |
||||||
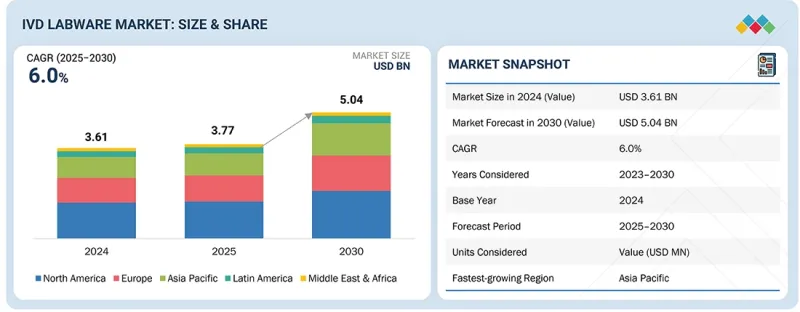
세계의 IVD 랩웨어 시장 규모는 2025년 약 37억 7,000만 달러에서 2030년까지 50억 4,000만 달러에 이를 것으로 예측됩니다.
예측 기간 동안 CAGR 6.0%로 성장할 전망입니다.
| 조사 범위 | |
|---|---|
| 조사 대상 기간 | 2024-2030년 |
| 기준 연도 | 2024년 |
| 예측 기간 | 2025-2030년 |
| 단위 | 금액(달러) |
| 부문 | 제품, 유형, 용도, 최종 사용자 |
| 대상 지역 | 북미, 유럽, 아시아태평양, 라틴아메리카, 중동 및 아프리카 |
진단 검사 수요가 꾸준히 증가하고 있는 것, 신뢰성이 높은 검체 처리에 대한 중시가 높아지고 있는 것, 임상 실험실 및 병원에서의 검사 서비스 확대가 진행되고 있는 등에 의해 시장은 강력한 성장을 보이고 있습니다. 질병의 조기 발견에 대한 의식 증가와 일관된 고품질 검사 워크플로우의 필요성은 IVD 랩웨어 수요를 더욱 강화하고 있습니다.

'제품 유형별로 플라스틱 부문이 예측 기간 동안 최대 CAGR을 나타낼 것으로 예상'
플라스틱 랩웨어 부문은 2024년에 가장 높은 성장을 기록했습니다. 이것은 광범위한 진단 워크플로우의 다양성을 지원합니다. 내구성, 일관된 제조 품질 및 일회용 검사 형식과의 호환성으로 실험실에서 널리 사용됩니다. 검사량 증가 및 워크플로우 표준화가 진행됨에 따라 플라스틱 랩웨어는 여전히 우선적으로 선택되는 제품으로 강한 시장 성장을 이끌고 있습니다.
'용도별로 분자 진단 부문이 예측 기간 동안 최대 성장을 보여줄 전망'
분자 진단 부문은 진보된 진단 기술의 보급 확대, 조기 및 정확한 질환 검출 수요 증가, 감염증 및 기타 진단 분야에서의 분자 검사 활용 확대 등을 바탕으로 예측 기간 동안 가장 높은 CAGR을 나타낼 것으로 전망됩니다.
'아시아태평양이 가장 빠르게 성장하고 있습니다.'
아시아태평양은 예측 기간 동안 가장 높은 성장률을 보일 것으로 예측됩니다. 이 성장은 신흥 경제국의 급속한 경제 발전, 가처분 소득 증가, 보다 광범위한 인구 기반의 의료 지출 확대로 뒷받침됩니다. 또한, 급성 및 만성 질환의 유병률 증가, 의료 인프라의 지속적인 현대화, 특히 준도시 및 농촌 지역에서 선진 진단 기술의 보급 확대가 IVD 랩웨어에 대한 수요를 이끌고 있습니다. 이러한 요인은 아시아태평양의 랩웨어 공급업체들에게 큰 시장 기회를 창출할 것으로 예측됩니다.
본 보고서에서는 세계의 IVD 랩웨어 시장을 조사했으며, 시장 개요, 시장 성장에 대한 각종 영향요인 분석, 기술 및 특허 동향, 법규제 환경, 사례 연구, 시장 규모 추이 및 예측, 각종 구분, 지역 및 주요 국가별 상세 분석, 경쟁 구도, 주요 기업 프로파일 등을 정리했습니다.
자주 묻는 질문
목차
제1장 서론
제2장 조사 방법
제3장 주요 요약
제4장 중요 인사이트
제5장 시장 개요
- 시장 역학
- 성장 촉진요인
- 성장 억제요인
- 기회
- 과제
- 언멧 요구 및 화이트 스페이스
- 관련 시장 및 이업종과의 분야 횡단적 기회
- Tier 1/2/3 기업의 전략적 움직임
제6장 업계 동향
- Porter's Five Forces 분석
- 거시경제지표
- 공급망 분석
- 밸류체인 분석
- 생태계 분석
- 가격 분석
- 무역 분석
- 주요 회의 및 이벤트(2025-2026년)
- 고객의 사업에 영향을 주는 동향 및 혁신
- 투자 및 자금조달 시나리오
- 사례 연구 분석
- 미국 관세가 IVD 랩웨어 시장에 미치는 영향(2025년)
제7장 기술의 진보, AI에 의한 영향, 특허, 혁신 및 장래의 용도
- 주요 신기술
- 면역 분석
- 분자 진단
- 임상 화학
- 미생물학
- 보완적 기술
- 마이크로 유체 공학 및 랩온칩 플랫폼
- 기술 및 제품 로드맵
- 특허 분석
- 미래의 용도
- AI 및 생성형 AI가 IVD 랩웨어 시장에 미치는 영향
제8장 지속가능성 및 규제 상황
- 지역 규제 및 규정 준수
- 지속가능성에 대한 노력
- 지속가능성에 미치는 영향 및 규제 정책의 노력
- 인증, 라벨, 환경 기준
제9장 고객 정세 및 구매 행동
- 의사결정 프로세스
- 주요 이해관계자 및 구매 기준
- 채용 장벽 및 내부 과제
- 다양한 최종 사용자 산업의 미충족 요구
- 시장 수익성
제10장 IVD 랩웨어 시장 : 제품별
- 플라스틱 랩웨어
- 튜브
- 플레이트
- 피펫
- 페트리 및 큐벳
- 기타
- 유리제 랩웨어
- 시험관
- 비커 및 플라스크
- 바이알
- 페트리 및 큐벳
- 슬라이드 및 커버 유리
- 기타
- 기타
제11장 IVD 랩웨어 시장 : 유형별
- 일회용 랩웨어
- 재사용 가능한 랩웨어
제12장 IVD 랩웨어 시장 : 용도별
- 면역 분석
- 임상 화학
- 분자 진단
- 혈액학
- 미생물학
- 기타
제13장 IVD 랩웨어 시장 : 최종 사용자별
- 병원
- 임상 실험실
- 학술기관
- 기타
제14장 IVD 랩웨어 시장 : 지역별
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타
- 아시아태평양
- 중국
- 일본
- 인도
- 기타
- 라틴아메리카
- 브라질
- 멕시코
- 기타 라틴아메리카
- 중동 및 아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
- 기타
제15장 IVD 랩웨어 시장 : 수량 분석
제16장 경쟁 구도
- 주요 진입 기업의 전략 및 강점
- 수익 분석
- 시장 점유율 분석
- 기업 평가 매트릭스 : 주요 기업
- 기업 평가 매트릭스 : 스타트업 및 중소기업
- 기업 평가 및 재무지표
- 브랜드 및 제품 비교
- 경쟁 시나리오
제17장 기업 프로파일
- 주요 기업
- THERMO FISHER SCIENTIFIC INC.
- GREINER AG
- AVANTOR, INC.
- CORNING INCORPORATED
- EPPENDORF SE
- TARSONS PRODUCTS LIMITED
- SARTORIUS AG
- SARSTEDT AG & CO. KG
- ABBOTT LABORATORIES
- BIOMERIEUX
- QIAGEN NV
- SYSMEX CORPORATION
- SCG PACKAGING PUBLIC COMPANY LIMITED
- 기타 기업
- AZENTA, INC.
- BRAND GROUP
- GENAXY SCIENTIFIC PVT. LTD.
- LABCON NORTH AMERICA
- ACCUMAX LAB DEVICES PVT. LTD.
- ZHEJIANG BIOLAND BIOTECHNOLOGY CO., LTD.
- ABDOS GROUP
- BEINGBIO
- WUXI NEST BIOTECHNOLOGY CO., LTD.
- BIOLYST SCIENTIFIC
- HEATHROW SCIENTIFIC
- KANGJIAN MEDICAL APPARATUS CO.
제18장 부록
AJY 25.12.17The market is valued at approximately USD 3.77 billion in 2025 and is projected to reach USD 5.04 billion by 2030, growing at a CAGR of 6.0% during the forecast period.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | Product, Type, Application, and End User |
| Regions covered | North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
The market is witnessing strong growth due to the steady rise in diagnostic testing needs, increasing emphasis on reliable sample handling, and the broader expansion of laboratory services across clinical laboratories and hospitals. Growing awareness of early disease detection and the need for consistent, high-quality testing workflows are further supporting the demand for IVD labware.
"Among product types, the plastic labware segment is projected to register the highest CAGR during the forecast period."
Based on product type, the global IVD labware market is segmented into plastic labware, glass labware, and other IVD labware products. In 2024, the plastic labware segment recorded the highest growth, supported by its versatility across a wide range of diagnostic workflows. Its durability, consistent manufacturing quality, and compatibility with single-use testing formats have made it widely used in laboratories. As testing volumes increase and workflows become more standardized, plastic labware remains the preferred choice, driving its strong market growth.
"By application, the molecular diagnostics segment is projected to register the highest growth during the forecasted period."
Based on application, the IVD labware market is segmented into immunoassays, clinical chemistry, molecular diagnostics, hematology, microbiology, and other applications. The molecular diagnostics segment is projected to register the highest CAGR during the forecast period, driven by the growing adoption of advanced diagnostic technologies, increasing demand for early and accurate disease detection, and the expanding use of molecular tests in infectious disease and other diagnostic areas.
"The Asia Pacific is the fastest-growing region in the IVD labware market."
The global IVD labware market is segmented into North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. Among these, the Asia Pacific region is expected to witness the highest growth during the forecast period. This growth is supported by rapid economic development in emerging economies, rising disposable incomes, and increased healthcare spending across a larger population base. Additionally, the growing prevalence of acute and chronic diseases, ongoing modernization of healthcare infrastructure, and wider adoption of advanced diagnostic technologies, especially in semi-urban and rural areas, are driving demand for IVD labware. These factors are anticipated to create substantial market opportunities for labware providers in the Asia Pacific region.
The break-up of the profile of primary participants in the IVD labware market:
- By Company Type: Tier 1 (40%), Tier 2 (30%), and Tier 3 (30%)
- By Designation: C-level Executives (28%), Director-level Executives (15%), and Others (57%)
- By Region: North America (51%), Europe (21%), Asia Pacific (18%), Latin America (6%), and the Middle East & Africa (4%)
The key players in this market are Thermo Fisher Scientific Inc. (US), Greiner AG (Austria), Avantor, Inc. (US), Corning Incorporated (US), Eppendorf SE (Germany), Tarsons Products Ltd. (India), Sartorius AG (Germany), QIAGEN N.V. (Netherlands), Abbott Laboratories (US), bioMerieux (France), Sysmex Corporation (Japan), SCG Packaging Public Company Limited (Thailand), Azenta, Inc. (US), GENAXY SCIENTIFIC PVT. LTD (India), SARSTEDT AG & Co. KG (Germany), Brand Group (Germany), Labcon North America (US), Accumax Lab Devices Pvt Ltd (India), Zhejiang Bioland Biotechnology Co., LTD (China), BeingBio (US), Wuxi NEST Biotechnology Co., Ltd. (China), BioVest Scientific (US), Heathrow Scientific (US), Abdos Group (India), and KANGJIAN Medical Apparatus Co.(China).
Research Coverage:
This research report categorizes the IVD labware market by product (plastic labware, glass labware, and other IVD labware products), by type (single-use labware and reusable labware), by application (immunoassays, clinical chemistry, molecular diagnostics, hematology, microbiology, and other applications), by end user (clinical laboratories, hospitals, academic institutes, and other end users), and region (North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa). The report's scope encompasses detailed information on the major factors, including drivers, restraints, opportunities, and challenges, which influence the growth of the IVD labware market. A detailed analysis of key industry players has been conducted to provide insights into their business overview, solutions, key strategies, including partnerships, collaborations, expansions, agreements, new product launches, and recent developments associated with the IVD labware market. This report provides a competitive analysis of upcoming startups in the IVD labware market ecosystem.
Reasons to buy this report:
The report will assist market leaders/new entrants in this market by providing information on the closest approximations of the revenue numbers for the overall IVD labware market and its subsegments. This report will help stakeholders understand the competitive landscape and gain valuable insights to better position their businesses and plan suitable go-to-market strategies. The report also helps stakeholders understand the pulse of the market, providing them with information on key market drivers, restraints, opportunities, and challenges.
The report provides insights into the following pointers:
- Analysis of key drivers (rising volume of diagnostic testing for chronic & infectious diseases, technological advancements in laboratories, expansion of diagnostic laboratories and healthcare infrastructure, and growing demand for advanced labware materials), opportunities (growing demand for sustainable and eco-friendly consumables and growth potential of emerging markets), restraints (high cost of advanced labware and environmental sustainability concerns), and challenges (need for regulatory compliance and lack of awareness and skills for advanced consumables) influencing the growth of the IVD labware market.
- Product Development/Innovation: Detailed insights on upcoming technologies, research & development activities, and new product launches in the IVD labware market.
- Market Development: Comprehensive information about lucrative markets; the report analyzes the IVD labware market across varied regions.
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the IVD labware market.
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and product offerings of leading players like Thermo Fisher Scientific Inc. (US), Greiner AG (Austria), Avantor, Inc. (US), Corning Incorporated (US), and Eppendorf SE (Germany), among others, in the IVD labware market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKETS COVERED & REGIONAL SCOPE
- 1.3.2 INCLUSIONS & EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.3.4 CURRENCY CONSIDERED
- 1.4 KEY STAKEHOLDERS
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.2 RESEARCH APPROACH
- 2.2.1 SECONDARY DATA
- 2.2.1.1 Key secondary sources
- 2.2.1.2 Key data from secondary sources
- 2.2.2 PRIMARY DATA
- 2.2.2.1 Primary sources
- 2.2.2.2 Key data from primary sources
- 2.2.2.3 Key industry insights
- 2.2.2.4 Breakdown of primary interviews
- 2.2.1 SECONDARY DATA
- 2.3 MARKET SIZE ESTIMATION
- 2.3.1 BOTTOM-UP APPROACH
- 2.3.1.1 Approach 1: Company revenue estimation approach
- 2.3.1.2 Approach 2: Presentations of companies and primary interviews
- 2.3.1.3 Growth forecast
- 2.3.1.4 CAGR projections
- 2.3.2 TOP-DOWN APPROACH
- 2.3.1 BOTTOM-UP APPROACH
- 2.4 MARKET BREAKDOWN & DATA TRIANGULATION
- 2.5 MARKET SHARE ASSESSMENT
- 2.6 RESEARCH ASSUMPTIONS
- 2.6.1 PARAMETRIC ASSUMPTIONS
- 2.7 RESEARCH LIMITATIONS
- 2.8 RISK ASSESSMENT
3 EXECUTIVE SUMMARY
- 3.1 KEY INSIGHTS & MARKET HIGHLIGHTS
- 3.2 KEY MARKET PARTICIPANTS: SHARE INSIGHTS & STRATEGIC DEVELOPMENTS
- 3.3 DISRUPTIVE TRENDS SHAPING MARKET GROWTH
- 3.4 HIGH-GROWTH SEGMENTS & EMERGING FRONTIERS
- 3.5 SNAPSHOT: GLOBAL MARKET SIZE, GROWTH RATE, AND FORECAST
4 PREMIUM INSIGHTS
- 4.1 IVD LABWARE MARKET OVERVIEW
- 4.2 IVD LABWARE MARKET, BY PRODUCT, 2025 VS. 2030 (USD MILLION)
- 4.3 IVD LABWARE MARKET, BY TYPE, 2025 VS. 2030 (USD MILLION)
- 4.4 IVD LABWARE MARKET, BY APPLICATION, 2025 VS. 2030 (USD MILLION)
- 4.5 IVD LABWARE MARKET, BY END USER, 2025 VS. 2030 (USD MILLION)
- 4.6 IVD LABWARE MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Rising volume of diagnostic testing for chronic & infectious diseases
- 5.2.1.2 Technological advancements in laboratories
- 5.2.1.3 Expansion of diagnostic laboratories and healthcare infrastructure
- 5.2.1.4 Growing demand for advanced labware materials
- 5.2.2 RESTRAINTS
- 5.2.2.1 High cost of advanced labware
- 5.2.2.2 Environmental sustainability concerns
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Growing demand for sustainable and eco-friendly consumables
- 5.2.3.2 Growth potential of emerging markets
- 5.2.4 CHALLENGES
- 5.2.4.1 Need for regulatory compliance
- 5.2.4.2 Lack of awareness and skills for advanced consumables
- 5.2.1 DRIVERS
- 5.3 UNMET NEEDS & WHITE SPACES
- 5.3.1 UNMET NEEDS IN IVD LABWARE MARKET
- 5.3.2 WHITE SPACE OPPORTUNITIES
- 5.4 INTERCONNECTED MARKETS & CROSS-SECTOR OPPORTUNITIES
- 5.4.1 INTERCONNECTED MARKETS
- 5.4.2 CROSS-SECTOR OPPORTUNITIES
- 5.5 STRATEGIC MOVES BY TIER 1/2/3 PLAYERS
- 5.5.1 KEY MOVES AND STRATEGIC FOCUS
6 INDUSTRY TRENDS
- 6.1 PORTER'S FIVE FORCES ANALYSIS
- 6.1.1 THREAT OF NEW ENTRANTS
- 6.1.2 THREAT OF SUBSTITUTES
- 6.1.3 BARGAINING POWER OF BUYERS
- 6.1.4 BARGAINING POWER OF SUPPLIERS
- 6.1.5 INTENSITY OF COMPETITIVE RIVALRY
- 6.2 MACROECONOMIC INDICATORS
- 6.2.1 INTRODUCTION
- 6.2.2 GDP TRENDS AND FORECAST
- 6.3 SUPPLY CHAIN ANALYSIS
- 6.4 VALUE CHAIN ANALYSIS
- 6.5 ECOSYSTEM ANALYSIS
- 6.5.1 IVD LABWARE MARKET: ROLE OF COMPANIES IN ECOSYSTEM
- 6.6 PRICING ANALYSIS
- 6.6.1 AVERAGE SELLING PRICE TREND OF IVD LABWARE, BY PRODUCT, 2023-2025
- 6.6.2 AVERAGE SELLING PRICE TREND OF PLASTIC LABWARE, BY KEY PLAYER, 2023-2025
- 6.6.3 AVERAGE SELLING PRICE TREND OF IVD LABWARE PRODUCTS, BY REGION, 2023-2025
- 6.7 TRADE ANALYSIS
- 6.7.1 IMPORT SCENARIO (HS CODE 7017)
- 6.7.2 EXPORT SCENARIO (HS CODE 7017)
- 6.8 KEY CONFERENCES & EVENTS, 2025-2026
- 6.9 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- 6.10 INVESTMENT & FUNDING SCENARIO
- 6.11 CASE STUDY ANALYSIS
- 6.11.1 CASE STUDY 1: IMPLEMENTATION OF CUSTOMIZED PIPETTING SYSTEM IN A MOLECULAR DIAGNOSTIC WORKFLOW
- 6.11.2 CASE STUDY 2: QUANTITATIVE COMPETITIVE PCR ASSAY FOR HIV-1 USING A MICROPLATE-BASED DETECTION APPROACH
- 6.11.3 CASE STUDY 3: MICROFLUIDIC PCR TUBE INTEGRATION FOR ON-TUBE PURIFICATION AND AMPLIFICATION
- 6.12 IMPACT OF 2025 US TARIFFS ON IVD LABWARE MARKET
- 6.12.1 INTRODUCTION
- 6.12.2 KEY TARIFF RATES
- 6.12.3 PRICE IMPACT ANALYSIS
- 6.12.4 KEY IMPACT ON COUNTRY/REGION
- 6.12.4.1 North America
- 6.12.4.2 Europe
- 6.12.4.3 Asia Pacific
- 6.12.5 IMPACT ON END-USE INDUSTRIES
- 6.12.5.1 Hospitals
- 6.12.5.2 Clinical laboratories
7 TECHNOLOGICAL ADVANCEMENTS, AI-DRIVEN IMPACT, PATENTS, INNOVATIONS, AND FUTURE APPLICATIONS
- 7.1 KEY EMERGING TECHNOLOGIES
- 7.1.1 IMMUNOASSAYS
- 7.1.2 MOLECULAR DIAGNOSTICS
- 7.1.3 CLINICAL CHEMISTRY
- 7.1.4 MICROBIOLOGY
- 7.2 COMPLEMENTARY TECHNOLOGIES
- 7.2.1 MICROFLUIDICS AND LAB-ON-A-CHIP PLATFORMS
- 7.3 TECHNOLOGY/PRODUCT ROADMAP
- 7.3.1 SHORT TERM (2025-2027) | PROCESS IMPROVEMENT & STANDARDIZATION
- 7.3.1.1 Focus areas:
- 7.3.1.1.1 Material development
- 7.3.1.1.2 Product innovations
- 7.3.1.1.3 Market adoption
- 7.3.1.1 Focus areas:
- 7.3.2 MID TERM (2027-2030) | MATERIAL DIVERSIFICATION & SUSTAINABILITY
- 7.3.2.1 Focus areas:
- 7.3.2.1.1 Material development
- 7.3.2.1.2 Product innovations
- 7.3.2.1.3 Market adoption
- 7.3.2.1 Focus areas:
- 7.3.3 LONG TERM (2030-2035+) | HIGH-PERFORMANCE & ECO-EFFICIENT LABWARE
- 7.3.3.1 Focus areas:
- 7.3.3.1.1 Material development
- 7.3.3.1.2 Product innovations
- 7.3.3.1.3 Market adoption
- 7.3.3.1 Focus areas:
- 7.3.1 SHORT TERM (2025-2027) | PROCESS IMPROVEMENT & STANDARDIZATION
- 7.4 PATENT ANALYSIS
- 7.4.1 LIST OF MAJOR PATENTS
- 7.5 FUTURE APPLICATIONS
- 7.6 IMPACT OF AI/GENERATIVE AI ON IVD LABWARE MARKET
- 7.6.1 INTRODUCTION
- 7.6.2 TOP USE CASES & MARKET POTENTIAL
- 7.6.3 AI USE CASES
- 7.6.4 IMPLEMENTATION OF AI, BY KEY COMPANY AND USE CASE
- 7.6.5 FUTURE OF AI IN IVD LABWARE MARKET
8 SUSTAINABILITY AND REGULATORY LANDSCAPE
- 8.1 REGIONAL REGULATIONS AND COMPLIANCE
- 8.1.1 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 8.1.2 INDUSTRY STANDARDS
- 8.2 SUSTAINABILITY INITIATIVES
- 8.2.1 ENVIRONMENTAL IMPACT AND ECO-FRIENDLY INITIATIVES IN IVD LABWARE
- 8.2.1.1 Eco-friendly Initiatives
- 8.2.1 ENVIRONMENTAL IMPACT AND ECO-FRIENDLY INITIATIVES IN IVD LABWARE
- 8.3 SUSTAINABILITY IMPACT & REGULATORY POLICY INITIATIVES
- 8.4 CERTIFICATIONS, LABELING, ECO-STANDARDS
9 CUSTOMER LANDSCAPE & BUYER BEHAVIOR
- 9.1 DECISION-MAKING PROCESS
- 9.2 KEY STAKEHOLDERS & BUYING EVALUATION CRITERIA
- 9.2.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 9.2.2 BUYING CRITERIA
- 9.3 ADOPTION BARRIERS & INTERNAL CHALLENGES
- 9.4 UNMET NEEDS IN VARIOUS END-USER INDUSTRIES
- 9.5 MARKET PROFITABILITY
- 9.5.1 REVENUE POTENTIAL
- 9.5.2 COST DYNAMICS
- 9.5.3 MARGIN OPPORTUNITIES IN KEY APPLICATIONS
10 IVD LABWARE MARKET, BY PRODUCT
- 10.1 INTRODUCTION
- 10.2 PLASTIC LABWARE
- 10.2.1 TUBES
- 10.2.1.1 Centrifuge/Microcentrifuge tubes
- 10.2.1.1.1 Growing demand for molecular and biochemical testing to drive adoption
- 10.2.1.2 PCR tubes
- 10.2.1.2.1 Growing adoption of molecular diagnostics to boost demand for PCR tubes
- 10.2.1.3 Cryovials/Storage vials
- 10.2.1.3.1 Rising need for secure sample preservation to support demand
- 10.2.1.4 Culture tubes
- 10.2.1.4.1 Rising focus on efficient sample cultivation to fuel demand
- 10.2.1.5 Other tubes
- 10.2.1.1 Centrifuge/Microcentrifuge tubes
- 10.2.2 PLATES
- 10.2.2.1 Microplates
- 10.2.2.1.1 Automation and standardization to support use of microplates
- 10.2.2.2 PCR plates
- 10.2.2.2.1 Rising incidence of infectious diseases to drive demand for PCR plates
- 10.2.2.3 Deep-well plates for sample preparation/extraction
- 10.2.2.3.1 Growing demand for high-throughput and automated sample handling to support growth
- 10.2.2.4 Cell culture plates
- 10.2.2.4.1 Increasing adoption of cell-based diagnostic methods to drive demand
- 10.2.2.5 Other plates
- 10.2.2.5.1 Increasing adoption of cell-based diagnostic methods to drive demand
- 10.2.2.1 Microplates
- 10.2.3 PIPETTES
- 10.2.3.1 Rising emphasis on accuracy in liquid handling to boost market demand
- 10.2.4 PETRI DISHES & CUVETTES
- 10.2.4.1 Growing incidence of infectious diseases and metabolic disorders to fuel adoption
- 10.2.5 OTHER PLASTIC LABWARE PRODUCTS
- 10.2.1 TUBES
- 10.3 GLASS LABWARE
- 10.3.1 TEST TUBES
- 10.3.1.1 Rising need for contamination-free sample handling to boost market growth
- 10.3.2 BEAKERS & FLASKS
- 10.3.2.1 Increased need for precise reagent preparation in diagnostics to drive adoption
- 10.3.3 VIALS
- 10.3.3.1 Rising focus on maintaining sample purity to boost demand
- 10.3.4 PETRI DISHES & CUVETTES
- 10.3.4.1 Increasing demand for precision and contamination control to support market growth
- 10.3.5 SLIDES & COVERSLIPS
- 10.3.5.1 Expanding use of microscopy in diagnostics to fuel adoption
- 10.3.6 OTHER GLASS LABWARE PRODUCTS
- 10.3.1 TEST TUBES
- 10.4 OTHER IVD LABWARE PRODUCTS
11 IVD LABWARE MARKET, BY TYPE
- 11.1 INTRODUCTION
- 11.2 SINGLE-USE LABWARE
- 11.2.1 NEED FOR EFFECTIVE INFECTION CONTROL AND PREVENTION OF CROSS-CONTAMINATION TO SUPPORT DEMAND
- 11.3 REUSABLE LABWARE
- 11.3.1 RISING INCIDENCE OF CHRONIC DISEASES TO PROVIDE OPPORTUNITIES FOR MARKET GROWTH
12 IVD LABWARE MARKET, BY APPLICATION
- 12.1 INTRODUCTION
- 12.2 IMMUNOASSAYS
- 12.2.1 EXPANDING USE OF IVD LABWARE IN IMMUNOASSAY TESTING TO SUPPORT MARKET GROWTH
- 12.3 CLINICAL CHEMISTRY
- 12.3.1 RISING INCIDENCE OF CHRONIC DISEASES TO PROVIDE OPPORTUNITIES FOR MARKET GROWTH
- 12.4 MOLECULAR DIAGNOSTICS
- 12.4.1 GROWING INCIDENCE OF INFECTIOUS DISEASES TO DRIVE DEMAND
- 12.5 HEMATOLOGY
- 12.5.1 INCREASING INCIDENCE OF BLOOD DISORDERS TO DRIVE ADOPTION OF IVD LABWARE
- 12.6 MICROBIOLOGY
- 12.6.1 INCREASING ADOPTION OF AUTOMATED CLINICAL MICROBIOLOGY TESTING INSTRUMENTS TO SUPPORT MARKET GROWTH
- 12.7 OTHER APPLICATIONS
13 IVD LABWARE MARKET, BY END USER
- 13.1 INTRODUCTION
- 13.2 HOSPITALS
- 13.2.1 LARGE VOLUME OF IVD TESTS PERFORMED TO SUPPORT MARKET GROWTH
- 13.3 CLINICAL LABORATORIES
- 13.3.1 EXPANDING DIAGNOSTIC WORKLOAD TO DRIVE LABWARE DEMAND
- 13.4 ACADEMIC INSTITUTES
- 13.4.1 EMPHASIS ON DIAGNOSTIC TRAINING IN ACADEMIC INSTITUTES TO BOOST MARKET GROWTH
- 13.5 OTHER END USERS
14 IVD LABWARE MARKET, BY REGION
- 14.1 INTRODUCTION
- 14.2 NORTH AMERICA
- 14.2.1 US
- 14.2.1.1 Presence of advanced healthcare infrastructure and high healthcare expenditures to drive the market
- 14.2.2 CANADA
- 14.2.2.1 Rising government initiatives to propel market growth
- 14.2.1 US
- 14.3 EUROPE
- 14.3.1 GERMANY
- 14.3.1.1 Increasing healthcare expenditure to drive market growth in Germany
- 14.3.2 UK
- 14.3.2.1 Growing number of accredited diagnostic & hospital laboratories to propel market
- 14.3.3 FRANCE
- 14.3.3.1 Increasing demand for early diagnosis to augment market growth
- 14.3.4 ITALY
- 14.3.4.1 Increased adoption of advanced diagnostic technologies and growing government healthcare investment to drive market
- 14.3.5 SPAIN
- 14.3.5.1 Growing disease burden and national initiatives to drive expansion of market
- 14.3.6 REST OF EUROPE
- 14.3.1 GERMANY
- 14.4 ASIA PACIFIC
- 14.4.1 CHINA
- 14.4.1.1 Growing access to modern healthcare to boost market growth
- 14.4.2 JAPAN
- 14.4.2.1 Well-developed healthcare system to drive market
- 14.4.3 INDIA
- 14.4.3.1 Expanding healthcare access and rising disease prevalence to drive market growth
- 14.4.4 REST OF ASIA PACIFIC
- 14.4.1 CHINA
- 14.5 LATIN AMERICA
- 14.5.1 BRAZIL
- 14.5.1.1 Universal healthcare access to drive adoption in Brazil
- 14.5.2 MEXICO
- 14.5.2.1 Improved access to healthcare to drive uptake of advanced diagnostic products
- 14.5.3 REST OF LATIN AMERICA
- 14.5.1 BRAZIL
- 14.6 MIDDLE EAST & AFRICA
- 14.6.1 SAUDI ARABIA
- 14.6.1.1 Rising government healthcare expenditure to boost market
- 14.6.2 UAE
- 14.6.2.1 Improvements in healthcare infrastructure to support growth
- 14.6.3 REST OF MIDDLE EAST & AFRICA
- 14.6.1 SAUDI ARABIA
15 IVD LABWARE MARKET: VOLUME ANALYSIS
- 15.1 INTRODUCTION
- 15.1.1 US: TOTAL NUMBER OF IVD TESTS CONDUCTED, 2023-2030 (MILLION)
16 COMPETITIVE LANDSCAPE
- 16.1 INTRODUCTION
- 16.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 16.2.1 OVERVIEW OF STRATEGIES DEPLOYED BY PLAYERS IN IVD LABWARE MARKET
- 16.3 REVENUE ANALYSIS, 2020-2024
- 16.4 MARKET SHARE ANALYSIS, 2024
- 16.4.1 GLOBAL MARKET SHARE ANALYSIS, 2024
- 16.4.2 US MARKET SHARE ANALYSIS, 2024
- 16.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 16.5.1 STARS
- 16.5.2 EMERGING LEADERS
- 16.5.3 PERVASIVE PLAYERS
- 16.5.4 PARTICIPANTS
- 16.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 16.5.5.1 Company footprint
- 16.5.5.2 Region footprint
- 16.5.5.3 Product footprint
- 16.5.5.4 Type footprint
- 16.5.5.5 Application footprint
- 16.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 16.6.1 PROGRESSIVE COMPANIES
- 16.6.2 RESPONSIVE COMPANIES
- 16.6.3 DYNAMIC COMPANIES
- 16.6.4 STARTING BLOCKS
- 16.6.5 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 16.6.5.1 Detailed list of key startups/SMEs
- 16.6.5.2 Competitive benchmarking of startups/SMEs (1/2)
- 16.6.5.3 Competitive benchmarking of startups/SMEs (2/2)
- 16.7 COMPANY VALUATION & FINANCIAL METRICS
- 16.7.1 FINANCIAL METRICS
- 16.7.2 COMPANY VALUATION
- 16.8 BRAND/PRODUCT COMPARISON
- 16.9 COMPETITIVE SCENARIO
- 16.9.1 PRODUCT LAUNCHES & APPROVALS
- 16.9.2 DEALS
- 16.9.3 EXPANSIONS
17 COMPANY PROFILES
- 17.1 KEY PLAYERS
- 17.1.1 THERMO FISHER SCIENTIFIC INC.
- 17.1.1.1 Business overview
- 17.1.1.2 Products offered
- 17.1.1.3 Recent developments
- 17.1.1.3.1 Deals
- 17.1.1.3.2 Expansions
- 17.1.1.4 MnM view
- 17.1.1.4.1 Key strengths
- 17.1.1.4.2 Strategic choices
- 17.1.1.4.3 Weaknesses & competitive threats
- 17.1.2 GREINER AG
- 17.1.2.1 Business overview
- 17.1.2.2 Products offered
- 17.1.2.3 Recent developments
- 17.1.2.3.1 Product approvals
- 17.1.2.4 MnM view
- 17.1.2.4.1 Key strengths
- 17.1.2.4.2 Strategic choices
- 17.1.2.4.3 Weaknesses & competitive threats
- 17.1.3 AVANTOR, INC.
- 17.1.3.1 Business overview
- 17.1.3.2 Products offered
- 17.1.3.3 Recent developments
- 17.1.3.3.1 Deals
- 17.1.3.3.2 Expansions
- 17.1.3.4 MnM view
- 17.1.3.4.1 Key strengths
- 17.1.3.4.2 Strategic choices
- 17.1.3.4.3 Weaknesses & competitive threats
- 17.1.4 CORNING INCORPORATED
- 17.1.4.1 Business overview
- 17.1.4.2 Products offered
- 17.1.4.3 Recent developments
- 17.1.4.3.1 Deals
- 17.1.4.4 MnM view
- 17.1.4.4.1 Key strengths
- 17.1.4.4.2 Strategic choices
- 17.1.4.4.3 Weaknesses & competitive threats
- 17.1.5 EPPENDORF SE
- 17.1.5.1 Business overview
- 17.1.5.2 Products offered
- 17.1.5.3 Recent developments
- 17.1.5.3.1 Product launches & approvals
- 17.1.5.3.2 Deals
- 17.1.5.3.3 Expansions
- 17.1.5.4 MnM view
- 17.1.5.4.1 Key strengths
- 17.1.5.4.2 Strategic choices
- 17.1.5.4.3 Weaknesses & competitive threats
- 17.1.6 TARSONS PRODUCTS LIMITED
- 17.1.6.1 Business overview
- 17.1.6.2 Products offered
- 17.1.7 SARTORIUS AG
- 17.1.7.1 Business overview
- 17.1.7.2 Products offered
- 17.1.7.3 Recent developments
- 17.1.7.3.1 Expansions
- 17.1.8 SARSTEDT AG & CO. KG
- 17.1.8.1 Business overview
- 17.1.8.2 Products offered
- 17.1.9 ABBOTT LABORATORIES
- 17.1.9.1 Business overview
- 17.1.9.2 Products offered
- 17.1.9.3 Recent developments
- 17.1.9.3.1 Deals
- 17.1.10 BIOMERIEUX
- 17.1.10.1 Business overview
- 17.1.10.2 Products offered
- 17.1.11 QIAGEN N.V.
- 17.1.11.1 Business overview
- 17.1.11.2 Products offered
- 17.1.11.3 Recent developments
- 17.1.11.3.1 Expansions
- 17.1.12 SYSMEX CORPORATION
- 17.1.12.1 Business overview
- 17.1.12.2 Products offered
- 17.1.12.3 Recent developments
- 17.1.12.3.1 Expansions
- 17.1.13 SCG PACKAGING PUBLIC COMPANY LIMITED
- 17.1.13.1 Business overview
- 17.1.13.2 Products offered
- 17.1.13.3 Recent developments
- 17.1.13.3.1 Deals
- 17.1.1 THERMO FISHER SCIENTIFIC INC.
- 17.2 OTHER PLAYERS
- 17.2.1 AZENTA, INC.
- 17.2.2 BRAND GROUP
- 17.2.3 GENAXY SCIENTIFIC PVT. LTD.
- 17.2.4 LABCON NORTH AMERICA
- 17.2.5 ACCUMAX LAB DEVICES PVT. LTD.
- 17.2.6 ZHEJIANG BIOLAND BIOTECHNOLOGY CO., LTD.
- 17.2.7 ABDOS GROUP
- 17.2.8 BEINGBIO
- 17.2.9 WUXI NEST BIOTECHNOLOGY CO., LTD.
- 17.2.10 BIOLYST SCIENTIFIC
- 17.2.11 HEATHROW SCIENTIFIC
- 17.2.12 KANGJIAN MEDICAL APPARATUS CO.
18 APPENDIX
- 18.1 DISCUSSION GUIDE
- 18.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 18.3 CUSTOMIZATION OPTIONS
- 18.4 RELATED REPORTS
- 18.5 AUTHOR DETAILS



















