
|
시장보고서
상품코드
1766265
인유두종 바이러스 백신 시장 : 기회, 성장 촉진요인, 산업 동향 분석, 예측(2025-2034년)Human Papillomavirus Vaccines Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
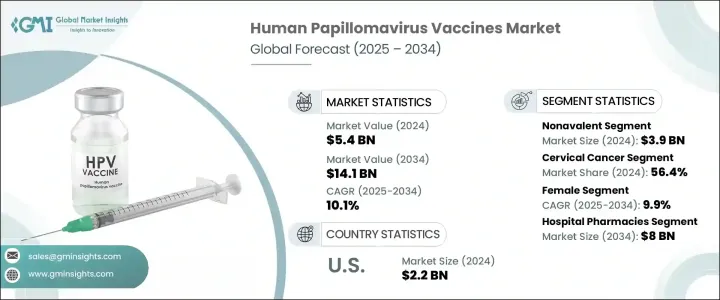
세계의 인유두종 바이러스 백신 시장은 2024년 54억 달러로 평가되었으며 CAGR 10.1%로 성장해 2034년까지 141억 달러에 이를 것으로 추정됩니다.
이 시장 확대는 자궁경부암, 항문암, 구강인두암, 기타 항문성기암의 원인인 HPV 감염의 세계 발생률 상승이 주요 요인입니다. 선진국과 개발도상국에서 HPV 관련 질환의 부담이 증가하고 있는 것과, 예방접종과 자궁경부암 치료를 널리 보급하기 위한 공중 위생의 대처가 함께, 큰 성장 요인이 되고 있습니다.
게다가 남성에서 HPV 감염의 유병률 증가나 성별에 얽매이지 않는 백신 접종 전략의 채용은 대상 인구를 확대하고 HPV 백신 수요를 밀어 올리고 있습니다. 게다가, 신흥 제조업체는 저렴한 가격의 백신을 제공해, 개발 도상 지역에 있어서 수요 증가에 대응하기 위해, 현지 생산에 주력하고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 54억 달러 |
| 예측 금액 | 141억 달러 |
| CAGR | 10.1% |
시장은 유형별로 2가 백신, 4가 백신, 비4가 백신으로 구분됩니다. 비원자가 백신은 9개의 고위험 HPV형(16, 18, 31, 33, 45, 52, 58)에 대한 방어를 제공해, 공중 위생의 중요한 요구에 대응하는 폭넓은 커버리지를 제공합니다.
2024년 시장 규모는 여성시장이 압도적으로 크고 CAGR 9.9%를 보일 것으로 예측됩니다. HPV 감염은 자궁경부암과 밀접한 관계가 있기 때문에 백신접종 프로그램은 주로 여성을 대상으로 하고 있으며, HPV 관련 질환의 발생률을 저하시키고 있습니다. 공공 캠페인과 NGO가 지원하는 여성의 건강에 대한 인식과 교육은 소녀와 젊은 여성의 백신 접종률을 높입니다.
미국의 인유두종 바이러스 백신 2024년 시장 규모는 22억 달러로 미국에서는 HPV 감염자 수가 증가하고 있으며, 예방 백신의 필요성이 높아지고 있습니다. 견고한 건강 관리 인프라, 예방 의료에 대한 정부 투자 증가가 시장 성장을 가속하는 주요 요인입니다.
인유두종 바이러스 백신 시장의 주요 기업은 Inovio Pharmaceuticals, Serum Institute of India, Recbio, GlaxoSmithKline, Merck, Walvax Biotechnology, Genexine, Sanofi Pasteur, Xiamen Innovax, Yuxi Zerun Biotech 등이 있습니다. HPV 백신 시장의 각사는 보다 광범위하고 장기적인 예방 효과를 제공하는 차세대 백신의 개발에 투자함으로써 기술 혁신에 주력하고 있습니다.
제조능력을 확대하고 유통망을 강화함으로써 특히 개발도상지역에서 세계 수요 증가에 대응하고 있습니다.
목차
제1장 조사 방법과 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 공급자의 상황
- 각 단계에서의 부가가치
- 밸류체인에 영향을 주는 요인
- 업계에 미치는 영향요인
- 성장 촉진요인
- HPV 관련 암의 이환율 상승
- 성별 중립적인 백신접종 정책으로의 이행이 증가
- 계발과 교육 캠페인 확대
- 백신 개발에서의 기술 진보
- 업계의 잠재적 위험 및 과제
- 첨단 백신의 고비용
- 집단 예방접종을 위한 인프라 부족
- 시장 기회
- 신흥 국가의 예방 접종 프로그램 확대
- 백신 배포에 있어서의 관민 파트너십
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 북미
- 유럽
- 아시아태평양
- 파이프라인 분석
- 미래 시장 동향
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 소개
- 기업의 시장 점유율 분석
- 기업 매트릭스 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 주요 발전
- 합병인수
- 파트너십 및 협업
- 신제품 발매
제5장 시장 추계 및 예측 : 유형별, 2021-2034년
- 주요 동향
- 2가
- 4가
- 9가
제6장 시장 추계 및 예측 : 적응증별, 2021-2034년
- 주요 동향
- 자궁경부암
- 질암 및 외음부암
- 항문암
- 중인두암
- 기타 적응증
제7장 시장 추계 및 예측 : 남녀별, 2021-2034년
- 주요 동향
- 여성
- 남성
제8장 시장 추계 및 예측 : 유통 채널별, 2021-2034년
- 주요 동향
- 병원 약국
- 소매 약국
- 온라인 약국
제9장 시장 추계 및 예측 : 지역별, 2021-2034년
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 프로파일
- Genexine
- GlaxoSmithKline
- Inovio Pharmaceuticals
- Merck
- Recbio
- Sanofi Pasteur
- Serum Institute of India
- Walvax Biotechnology
- Xiamen Innovax
- Yuxi Zerun Biotech
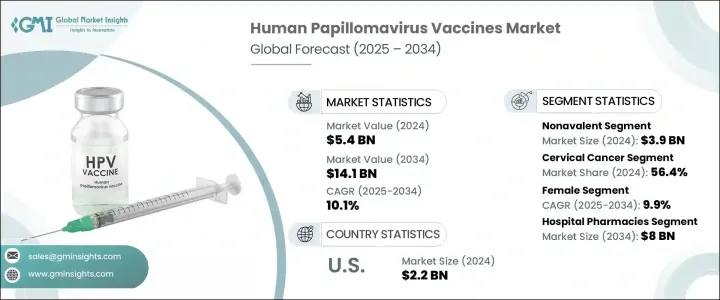
The Global Human Papillomavirus Vaccines Market was valued at USD 5.4 billion in 2024 and is estimated to grow at a CAGR of 10.1% to reach USD 14.1 billion by 2034. This expansion is largely driven by the rising incidence of HPV infections, which contribute to cervical, anal, oropharyngeal, and other anogenital cancers worldwide. Increasing awareness about HPV-related cancers and the importance of vaccination programs is further propelling market growth. The rising burden of HPV-associated diseases in both developed and developing countries, coupled with public health efforts to promote widespread immunization and cervical cancer treatment, is a significant growth factor.
Additionally, the increasing prevalence of HPV infections in men and the adoption of gender-neutral vaccination strategies are broadening the target population and boosting demand for HPV vaccines. Advances in vaccine technology, such as recombinant DNA techniques and virus-like particle platforms, are leading to the development of next-generation vaccines that offer broader protection and longer-lasting immunity. Moreover, emerging manufacturers are focusing on local production to provide affordable vaccines and meet growing demand in developing regions. The HPV vaccines market is centered around the development, manufacture, and distribution of vaccines that prevent HPV infections, primarily delivered through immunization programs targeting adolescents and young adults.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $5.4 Billion |
| Forecast Value | $14.1 Billion |
| CAGR | 10.1% |
The market is segmented by type into bivalent, quadrivalent, and nonavalent vaccines. The nonavalent vaccine segment held the largest share in 2024, valued at USD 3.9 billion. Increased awareness of HPV-related cancers has led healthcare providers and governments to prioritize vaccines targeting the most oncogenic HPV strains. The nonavalent vaccine offers protection against nine high-risk HPV types (16, 18, 31, 33, 45, 52, and 58), providing a wider coverage that addresses critical public health needs. Government immunization initiatives have played a pivotal role in the increased adoption of nonavalent vaccines.
The female segment dominated the market in 2024 and is anticipated to grow at a CAGR of 9.9%. Since HPV infection is closely linked to cervical cancer, vaccination programs primarily target females to reduce the incidence of HPV-related diseases. Public health policies and international recommendations have emphasized female immunization, which has significantly boosted demand. Enhanced awareness and education around women's health, supported by public campaigns and NGOs, have increased vaccine uptake among girls and young women. The growing involvement of parents and caregivers has also reinforced the dominance of this segment.
U.S. Human Papillomavirus Vaccines Market was valued at USD 2.2 billion in 2024. The rising number of HPV cases in the country drives the need for preventive vaccines. Public health initiatives, a high disease burden, robust healthcare infrastructure, and increased government investments in preventive healthcare are key factors fueling market growth. Gender-neutral vaccination policies and school-based immunization programs continue to push demand within the U.S.
Key players in the Human Papillomavirus Vaccines Market include Inovio Pharmaceuticals, Serum Institute of India, Recbio, GlaxoSmithKline, Merck, Walvax Biotechnology, Genexine, Sanofi Pasteur, Xiamen Innovax, and Yuxi Zerun Biotech. Companies in the HPV vaccines market focus on innovation by investing in the development of next-generation vaccines that provide broader and longer-lasting protection. Strategic partnerships with research institutions and biotechnology firms enable accelerated product development and regulatory approvals.
Expanding manufacturing capacities and enhancing distribution networks help meet rising global demand, especially in developing regions. Firms are also prioritizing affordability and accessibility by collaborating with local manufacturers to reduce costs. Additionally, targeted awareness campaigns and advocacy efforts promote vaccine adoption, supporting market growth and strengthening their presence worldwide.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumption and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Type
- 2.2.3 Indication
- 2.2.4 Gender
- 2.2.5 Distribution channel
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of HPV-related cancers
- 3.2.1.2 Increasing shift toward gender-neutral vaccination policies
- 3.2.1.3 Growing awareness and education campaigns
- 3.2.1.4 Technological advancements in vaccine development
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of advanced vaccines
- 3.2.2.2 Lack of infrastructure for mass immunization
- 3.2.3 Market opportunities
- 3.2.3.1 Expanding immunization programs in developing countries
- 3.2.3.2 Public-private partnerships in vaccine distribution
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Pipeline analysis
- 3.6 Future market trends
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
Chapter 5 Market Estimates and Forecast, By Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Bivalent
- 5.3 Quadrivalent
- 5.4 Nonavalent
Chapter 6 Market Estimates and Forecast, By Indication, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Cervical cancer
- 6.3 Vaginal and vulvar cancer
- 6.4 Anal cancer
- 6.5 Oropharyngeal cancer
- 6.6 Other indications
Chapter 7 Market Estimates and Forecast, By Gender, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Female
- 7.3 Male
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospital pharmacies
- 8.3 Retail pharmacies
- 8.4 Online pharmacies
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Genexine
- 10.2 GlaxoSmithKline
- 10.3 Inovio Pharmaceuticals
- 10.4 Merck
- 10.5 Recbio
- 10.6 Sanofi Pasteur
- 10.7 Serum Institute of India
- 10.8 Walvax Biotechnology
- 10.9 Xiamen Innovax
- 10.10 Yuxi Zerun Biotech


















