
|
시장보고서
상품코드
1851830
인유두종바이러스 백신 시장 : 점유율 분석, 산업 동향, 통계, 성장 예측(2025-2030년)Human Papillomavirus Vaccine - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
세계의 인유두종바이러스 백신 시장 규모는 2025년 89억 8,000만 달러로, 2030년까지 135억 1,000만 달러에 이르고, CAGR 8.51%로 성장할 전망입니다.

2024년 후반에 세계보건기구(WHO)가 승인한 단회 투여 스케줄, 저소득 및 중소득국(LMIC) 제조업체의 급증, 젠더 중립 정책의 확대에 의해 임상 수요 패턴이 재정의되고 있습니다. 비 원자가 제제의 급속한 보급, 인도, 중국, 유럽 연합(EU)에서의 대규모 정부 자금 기여, 두경부암을 커버하는 FDA 라벨의 확대가 함께 견조한 수량 성장을 지원하고 있습니다. 제조업체 각 회사는 동시에 단일 용량 요법 및 지역 특이적 다가 제형 플랫폼을 위한 생산 체제를 재구성하고 있는 반면, 지불자는 마진을 손상시키지 않고 액세스를 확대하기 위한 단계적 가격 설정을 협상하고 있습니다. 이러한 힘을 통해 인유두종바이러스 백신 시장은 세계의 공공 프로그램과 프리미엄 민간 부문에서 지속적인 확장을 기대할 수 있습니다.
세계의 인유두종바이러스 백신 시장 동향과 통찰
새로운 다가 HPV 백신 승인
두경부암 예방을 위한 Gardasil 9의 최근 FDA 승인은 보호 범위를 부인과 악성 종양에 그치지 않고 남성 성인 분야로도 확대했습니다. Merck와 아시아의 개발 기업 여러 회사는 아프리카와 동남아시아에서 매우 유행하는 HPV형을 대상으로 차세대 다가 후보를 추진하고 있으며, 인유두종바이러스 백신 시장의 프리미엄층을 강화하고 있습니다. WHO의 파이프라인 검토는 20가지 이상의 치료용 백신을 나열하여 예방과 치료가 향후 융합될 것임을 보여줍니다. 이러한 혁신은 전반적으로 판매량 증가를 지원하고 지역 간 가격 차별화를 촉진합니다.
정부와 다자간 자금 지원을 통한 가속기
인도의 2024년도 예산은 현지에서 생산된 Cervavac을 사용하는 여아용 국가 프로그램에 민간 기업에 의한 1회 접종량당 24달러로 자금을 제공해 인유두종바이러스 백신 시장에서 최대 규모의 단일 확대를 실현했습니다. Gavi의 6억 달러 약속과 2013년 이후 유니세프의 총 조달량이 9,300만 회분을 넘어서 아시아와 라틴아메리카에서의 능력 증강이 정당화되는 안정적인 수요가 예측됩니다. 2025년에는 PAHO 하에서 유사한 협정이 맺어지며, 지역별 주문이 더욱 집계되므로 단가가 낮아지고 접근성이 확대됩니다.
엄격한 생물 제제 규제
복잡한 생물제제의 틀은 철저한 안전성과 효력 데이터를 요구하고, 개발 비용을 후보품 1개당 10억 달러 이상으로 밀어 올리고, 지역 제조업체의 승인을 늦추고 있습니다. 유럽 의약품청(EEA)의 신청 서류는 또 다른 단계 컴플라이언스를 강화하고 WHO의 사전 승인은 다자간 입찰에 필수적입니다. 이러한 계층은 시장 출시 시간을 늘리고 자본력 있는 기존 기업에 유리하며 인유두종바이러스 백신 시장에서 소규모 진출기업의 성장을 억제합니다.
부문 분석
4가 백신은 2024년에 67.53%의 점유율을 유지하여 국가 프로그램에 대한 광범위한 침투를 지원합니다. 4가 백신의 지위는 확립되어 예측 가능한 수익 흐름을 지원하고 있지만, 고가의 비 4가 백신이 CAGR 9.24%로 웃돌고 있습니다. 비 원자가 제품의 인유두종바이러스 백신 시장 규모는 북미와 유럽에서 강력하게 확대되고 있습니다. 이러한 변화는 중소득층 소비자가 보다 광범위한 보호를 위해 돈을 지불하고자 하는 반면, 중소득층 조달 기관은 비용 효율적인 4가 또는 2가 용량을 계속 구매한다는 장점도 있습니다.
세계적인 임상 증거에 따르면, Gardasil 9는 거의 전 세계의 자궁 경부암을 예방하고, 다른 HPV 관련 질환에 대해서는 90%의 효능을 나타냅니다. WHO가 Cecolin을 1회 접종용으로, Walrinvax를 2회 접종용으로 사전 승인함으로써 공급 옵션이 넓어져 인유두종바이러스 백신 시장의 가격대를 압박하고 있습니다. 미래에는 아프리카와 아시아에서 유행하는 균주용으로 설계된 지역 특이적 다가 후보 약물이 경쟁 분야를 더욱 세분화할 수 있습니다.
자궁경부암 예방은 공중위생에 대한 관심의 정착과 수십년에 걸친 데이터의 뒷받침으로 2024년 매출의 69.98%를 차지했습니다. 그러나 항문암 예방은 CAGR 9.31%로 가장 빠르게 증가하고 있습니다. 이것은 성별에 얽매이지 않는 정책이 남성의 질병 부담 증가를 인식하고 있기 때문입니다. 인유두종바이러스 백신의 자궁 경부암 치료제 시장 점유율은 자궁 경부암 이외의 적응증이 주목됨에 따라 점차 감소하고 있지만, 절대적인 매출은 계속 성장하고 있습니다.
FDA에 의한 Gardasil 9의 두경부암에 대한 적응 확대는 여성 중심의 적응을 넘어서는 확대를 검증하여 성인 캐치업 프로그램에 반영합니다. 검토된 데이터는 남성의 중인두암 이환율이 상승하고 있는 것으로 밝혀져 세계 정책이 갱신되는 계기가 되었습니다. 따라서 제조업체는 양성에 호소하는 종합적인 암 예방을 위한 가치 제안을 재배치하고 있습니다.
지역 분석
북미 점유율 39.48%는 조기 도입, 광범위한 보험 적용, 지속적인 성별에 얽매이지 않는 캐치업 이니셔티브를 반영합니다. 미국은 아직 지불에 연동한 격차를 볼 수 있지만, 캐나다는 2024년에 9-20세를 대상으로 1회 접종을 추천하고 있어 정책의 선구가 되고 있습니다. 멕시코가 PAHO의 배제 로드맵에 참여하면 전체 서브리전 가격 및 조달 시너지 효과가 기대됩니다.
아시아태평양은 CAGR 9.22%에서 성장을 이끌고 있으며, 이는 인도의 여아 프로그램에 대한 전액 출자와 구미의 기존 기업에 과제하는 중국의 국내 생산자의 급증에 추진되고 있습니다. Merck의 중국 2025년 1분기 매출이 41% 감소한 것은 가격 경쟁의 격화와 규제의 복잡성을 돋보이게 합니다. 일본의 9가 백신으로의 전환은 호주의 성숙한 배제 전략과 함께 지역 전체의 인유두종바이러스 백신 시장 규모를 확대하는 정책 모델의 다양성을 보여줍니다.
유럽에서는 2,000만 유로의 EU4Health 자금을 바탕으로 여성의 접종률 90%, 남성의 접종 의무 확대라는 정책 혁신을 계속하고 있습니다. 경쟁 입찰은 공급의 안정성을 손상시키지 않고 저렴한 가격을 유지합니다. 중동 및 아프리카는 콜드체인과 주저 장벽에 직면하고 있지만, 나이지리아에서는 2024년부터 2025년까지 여아 770만명으로의 백신 접종을 추진하고 있어 지역 중심의 대처가 높은 이용률을 확보할 수 있음을 나타내고 있습니다. 남미에서는 PAHO와 스페인 기관과의 2025년 파트너십을 통해 9가 백신에 대한 접근성을 강화할 것입니다.
기타 혜택:
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간의 애널리스트 서포트
목차
제1장 서론
- 조사의 전제조건과 시장의 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 상황
- 시장 개요
- 시장 성장 촉진요인
- 새로운 다가 HPV 백신 승인
- 정부와 다자간 자금 지원에 의한 가속기
- 성별에 얽매이지 않는 예방접종 정책
- HPV와 관련된 암 이환율의 상승
- WHO가 승인한 1회 투여 스케줄
- LMIC에 거점을 두는 백신 제조업체의 출현
- 시장 성장 억제요인
- 엄격한 생물 제제 규제
- 예방 접종에 대한 주저와 잘못된 정보
- 높은 Mics 조달 비용
- 중소득국에서의 콜드체인과 라스트 마일 갭
- 규제 상황
- Porter's Five Forces 분석
- 공급기업의 협상력
- 구매자의 협상력
- 신규 참가업체의 위협
- 대체품의 위협
- 경쟁 기업간 경쟁 관계
제5장 시장 규모와 성장 예측
- 백신 유형별
- 2가
- 4가
- 9가
- 적응증별
- 자궁경부암
- 항문암
- 남근암
- 구인두암
- 성기 사마귀
- 기타
- 유통 채널별
- 공공
- 민간
- 연령층별
- 성인
- 소아
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 시장 집중도
- 시장 점유율 분석
- 기업 프로파일
- Merck & Co., Inc.
- GSK plc
- Serum Institute of India Pvt Ltd
- Walvax Biotechnology Co. Ltd
- Bharat Biotech
- Innovax(Xiamen Innovax Biotech)
- Wantai BioPharm
- Sinovac Biotech Ltd
- INOVIO Pharmaceuticals
- Novartis AG
- AstraZeneca plc
- Dynavax Technologies
- CSL Seqirus
- Shenzhen Kangtai Biological
- Pfizer Inc.
- Sanofi SA
- VBI Vaccines
- Geneos Therapeutics
- Takeda Pharmaceutical
- Daiichi-Sankyo
제7장 시장 기회와 장래의 전망
JHS 25.11.25The human papillomavirus vaccine market is valued at USD 8.98 billion in 2025 and is on track to reach USD 13.51 billion by 2030, advancing at an 8.51% CAGR.
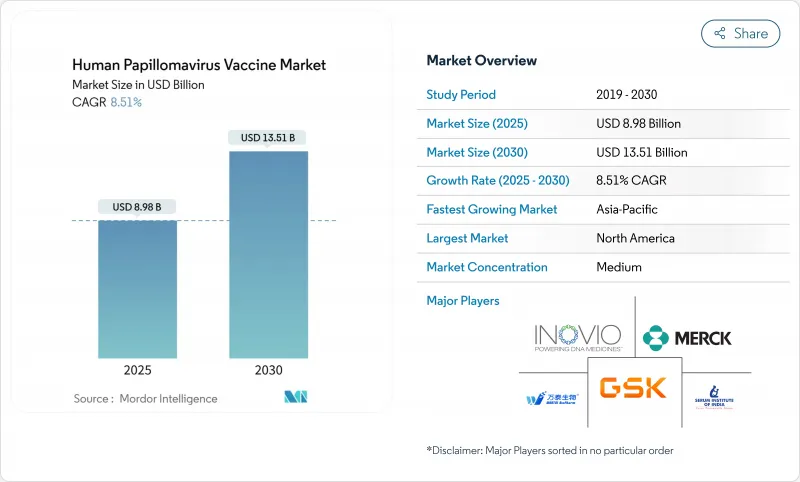
Single-dose schedules endorsed by the World Health Organization (WHO) in late-2024 , a surge of low- and middle-income country (LMIC) manufacturers, and widening gender-neutral policies are redefining clinical demand patterns. The rapid uptake of nonavalent formulations, large-scale government funding commitments in India, China and the European Union, and the extension of FDA labels to cover head-and-neck cancers together sustain robust volume growth. Manufacturers are simultaneously re-tooling production for single-dose regimens and region-specific multivalent platforms, while payers negotiate tiered pricing that broadens access without eroding margins. These forces position the human papillomavirus vaccine market for sustained expansion across public programs and premium private segments worldwide.
Global Human Papillomavirus Vaccine Market Trends and Insights
Approval of New Multivalent HPV Vaccines
Recent FDA clearance of Gardasil 9 for head-and-neck cancer prevention extends protective reach beyond gynecological malignancies and opens male adult segments. Merck and several Asian developers are advancing next-generation multivalent candidates aimed at HPV types highly prevalent in Africa and South-East Asia, reinforcing the premium tier of the human papillomavirus vaccine market. WHO's pipeline review lists more than 20 therapeutic vaccines, signalling future convergence of preventive and therapeutic modalities . Collectively, these innovations underpin volume growth and encourage price differentiation across regions.
Government & Multi-Lateral Funding Accelerators
India's 2024 budget funded a national girls' program using locally produced Cervavac at USD 24 per private-sector dose, creating the largest single expansion in the human papillomavirus vaccine market. Gavi's USD 600 million commitment and UNICEF's aggregate procurement surpassing 93 million doses since 2013 provide stable demand forecasts that justify capacity builds in Asia and Latin America . Similar agreements under PAHO in 2025 further aggregate regional orders, lowering unit cost and widening access.
Stringent Biologics Regulations
Complex biologics frameworks require exhaustive safety and potency data, driving development costs above USD 1 billion per candidate and delaying approvals for regional manufacturers. European Medicines Agency dossiers add another tier of compliance, while WHO prequalification remains essential for multilateral tenders. These layers heighten time-to-market and favor incumbents with deep capital reserves, tempering the growth of smaller entrants in the human papillomavirus vaccine market.
Other drivers and restraints analyzed in the detailed report include:
- Gender-Neutral Immunization Policies
- One-Dose Schedule Endorsed By WHO
- Vaccine Hesitancy & Misinformation
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Quadrivalent vaccines retained 67.53% share in 2024, underpinning broad national program penetration. Their entrenched status supports predictable revenue flow, yet premium-priced nonavalent products are outpacing at a 9.24% CAGR. The human papillomavirus vaccine market size for nonavalent offerings is expanding strongly in North America and Europe as payers endorse wider oncogenic strain coverage. This shift also benefits middle-income consumers willing to pay for broader protection, even while procurement agencies in LMICs continue purchasing cost-efficient quadrivalent or bivalent doses.
Global clinical evidence shows Gardasil 9 delivering near-universal cervical cancer protection and 90% efficacy against other HPV-related conditions. WHO prequalification of Cecolin for single-dose schedules and Walrinvax for two-dose regimes broadens supply options, pressuring price points in the human papillomavirus vaccine market. Looking forward, region-specific multivalent candidates designed for African and Asian strain prevalence may further fragment the competitive field.
Cervical cancer prevention accounted for 69.98% of 2024 revenue thanks to entrenched public-health focus and decades of supportive data. Yet anal cancer prevention is rising fastest at 9.31% CAGR because gender-neutral policies recognize growing male disease burden. The human papillomavirus vaccine market share for cervical applications will gradually erode as non-cervical indications gain prominence, though absolute revenues continue growing.
FDA extension of Gardasil 9 to head-and-neck cancers validates expansion beyond female-centric indications and informs adult catch-up programs. Peer-reviewed data reveal a rising incidence of oropharyngeal cancer among men, catalyzing policy updates worldwide. Manufacturers are therefore repositioning value propositions toward comprehensive cancer prophylaxis that appeals to both sexes.
The Human Papillomavirus Vaccine Market is Segmented by Vaccine Type (Bivalent, Quadrivalent, and More), Indication (Cervical Cancer, Anal Cancer, Penile Cancer, and More), Distribution Channel (Public and Private), Age Group (Adults and Pediatric) and Geography (North America, Europe, Asia-Pacific, and More). The Market and Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America's 39.48% share reflects early adoption, broad insurance coverage, and ongoing gender-neutral catch-up initiatives. The United States still shows payment-linked gaps, yet Canada's 2024 single-dose recommendation for those aged 9-20 positions it as a policy bellwether. Mexico's participation in PAHO's elimination roadmap promises synergies in pricing and procurement across the sub-region.
Asia-Pacific leads growth at a 9.22% CAGR, propelled by India's fully funded girls' program and China's surge of domestic producers challenging Western incumbents. Merck's 41% Q1-2025 sales decline in China highlights intensifying price competition and regulatory complexity. Japan's shift to 9-valent vaccines for boys and girls, together with Australia's mature elimination strategy, illustrates the diversity of policy models that collectively enlarge the human papillomavirus vaccine market size across the region.
Europe continues policy innovation through its 90% female coverage and expanding male vaccination mandate, underwritten by EUR 20 million in EU4Health funds. Competitive tenders sustain affordability without compromising supply security. The Middle East and Africa face cold-chain and hesitancy barriers, yet Nigeria's 2024-2025 drive to reach 7.7 million girls shows that community-centric engagement can secure high utilization. In South America, PAHO's 2025 partnership with Spanish agencies enhances access to 9-valent vaccines, while differential economics across the continent necessitate phased rollouts.
- Merck
- GlaxoSmithKline
- Serum Institute of India Pvt Ltd
- Walvax Biotechnology Co. Ltd
- Bharat Biotech
- Innovax (Xiamen Innovax Biotech)
- Wantai BioPharm
- Sinovac Biotech
- INOVIO Pharmaceuticals
- Novartis
- AstraZeneca
- Dynavax Technologies
- CSL Seqirus
- Shenzhen Kangtai Biological
- Pfizer
- Sanofi
- VBI Vaccines
- Geneos Therapeutics
- Takeda Pharmaceuticals
- Daiichi-Sankyo
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Approval of New Multivalent HPV Vaccines
- 4.2.2 Government & Multi-Lateral Funding Accelerators
- 4.2.3 Gender-Neutral Immunization Policies
- 4.2.4 Rising HPV-Linked Cancer Incidence
- 4.2.5 One-Dose Schedule Endorsed By WHO
- 4.2.6 Emergence of LMIC-Based Vaccine Manufacturers
- 4.3 Market Restraints
- 4.3.1 Stringent Biologics Regulations
- 4.3.2 Vaccine Hesitancy & Misinformation
- 4.3.3 High Procurement Cost for Mics
- 4.3.4 Cold-Chain & Last-Mile Gaps in LMICs
- 4.4 Regulatory Landscape
- 4.5 Porters Five Forces Analysis
- 4.5.1 Bargaining Power of Suppliers
- 4.5.2 Bargaining Power of Buyers
- 4.5.3 Threat of New Entrants
- 4.5.4 Threat of Substitutes
- 4.5.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Vaccine Type
- 5.1.1 Bivalent
- 5.1.2 Quadrivalent
- 5.1.3 Nonavalent
- 5.2 By Indication
- 5.2.1 Cervical Cancer
- 5.2.2 Anal Cancer
- 5.2.3 Penile Cancer
- 5.2.4 Oropharyngeal Cancer
- 5.2.5 Genital Warts
- 5.2.6 Others
- 5.3 By Distribution Channel
- 5.3.1 Public
- 5.3.2 Private
- 5.4 By Age Group
- 5.4.1 Adults
- 5.4.2 Pediatric
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global-level Overview, Market-level Overview, Core Segments, Financials, Strategic Information, Market Rank/Share, Products & Services, Recent Developments)
- 6.3.1 Merck & Co., Inc.
- 6.3.2 GSK plc
- 6.3.3 Serum Institute of India Pvt Ltd
- 6.3.4 Walvax Biotechnology Co. Ltd
- 6.3.5 Bharat Biotech
- 6.3.6 Innovax (Xiamen Innovax Biotech)
- 6.3.7 Wantai BioPharm
- 6.3.8 Sinovac Biotech Ltd
- 6.3.9 INOVIO Pharmaceuticals
- 6.3.10 Novartis AG
- 6.3.11 AstraZeneca plc
- 6.3.12 Dynavax Technologies
- 6.3.13 CSL Seqirus
- 6.3.14 Shenzhen Kangtai Biological
- 6.3.15 Pfizer Inc.
- 6.3.16 Sanofi SA
- 6.3.17 VBI Vaccines
- 6.3.18 Geneos Therapeutics
- 6.3.19 Takeda Pharmaceutical
- 6.3.20 Daiichi-Sankyo
7 Market Opportunities & Future Outlook
- 7.1 White-space & unmet-need assessment
















