
|
시장보고서
상품코드
1800739
인간 마이크로바이옴 시장 : 제품별, 유형별, 질환별, 투여 경로별, 서비스별, 최종사용자별, 지역별 - 예측(-2031년)Human Microbiome Market by Product (Drugs, Probiotics, Prebiotics, Synbiotics, Diagnostics), Disease (Infectious, Gastrointestinal), Route (Oral, Rectal), CDMO (Formulation, Strain Engineering), Type (BCT/FMT, Live Bacteria) - Global Forecast to 2031 |
||||||
인간 마이크로바이옴 시장 규모는 예측 기간 동안 31.0%의 CAGR로 확대되어 2024년 14억 달러에서 2031년에는 70억 9,000만 달러에 달할 것으로 예측됩니다.
| 조사 범위 | |
|---|---|
| 조사 대상 연도 | 2024-2031년 |
| 기준 연도 | 2024년 |
| 예측 기간 | 2025-2031년 |
| 검토 단위 | 금액(10억 달러) |
| 부문 | 제품별, 유형별, 질환별, 투여 경로별, 서비스별, 최종사용자별, 지역별 |
| 대상 지역 | 북미, 유럽, 아시아태평양, 라틴아메리카, 중동 및 아프리카 |
마이크로바이옴 연구를 위한 마이크로바이옴 산업계와 학계의 협력 체계와 맞춤형 의료에 대한 수요 증가 등의 요인이 인간 마이크로바이옴 시장의 성장을 촉진하고 있습니다. 그러나 복잡한 규제 정책이 마이크로바이옴의 상업화에 부정적인 영향을 미쳐 시장 성장을 저해하고 있습니다.
2024년 인간 마이크로바이옴 보충제 분야에서는 프로바이오틱스 분야가 가장 큰 점유율을 차지했습니다.
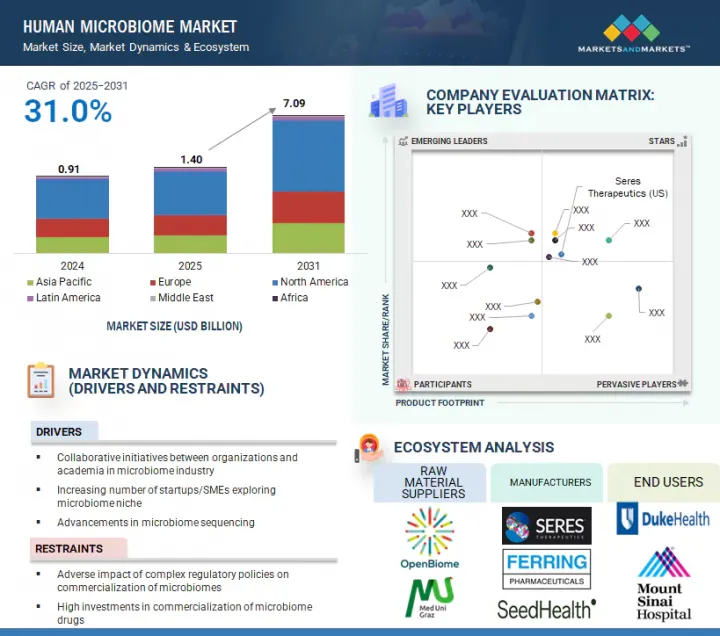
시장은 인간 마이크로바이옴 의약품, 인간 마이크로바이옴 보충제, 인간 마이크로바이옴 진단으로 구분됩니다. 인간 마이크로바이옴 보충제는 프로바이오틱스, 프리바이오틱스, 신바이오틱스 등 3가지 주요 제품으로 분석됩니다. 2024년 인간 마이크로바이옴 시장에서 가장 큰 점유율을 차지한 부문은 프로바이오틱스입니다. 이러한 우위는 프로바이오틱스의 광범위한 사용, 소비자의 높은 인지도, 시판 중인 건강 및 웰빙 제품에서 확고한 입지를 구축한 덕분입니다. 프로바이오틱스는 소화기 건강을 지원하고 면역력을 강화하며 장내 미생물의 균형을 유지하기 위해 일반적으로 사용되며, 예방 건강 관리의 일상적인 부분으로 자리 잡았습니다. 프로바이오틱스는 캡슐, 분말, 드링크제, 기능성 식품 등 다양한 형태로 판매되고 있으며, 쉽게 구할 수 있어 소비자들의 지지를 받고 있습니다. 또한, 많은 임상 연구가 특정 프로바이오틱스 균주의 효능을 입증하여 소비자와 의료 서비스 제공자의 신뢰도를 높이고 있습니다. 프로바이오틱스를 프리바이오틱스, 비타민 등 다른 생리활성물질과 결합할 수 있기 때문에 다양한 계층에서 프로바이오틱스의 매력이 더욱 확대되고 있습니다. 이러한 요인들이 복합적으로 작용하여 프로바이오틱스는 인간 마이크로바이옴 보충제 분야의 주요 제품 유형이 되었습니다.
인체 마이크로바이옴 시장은 소화기 질환, 감염성 질환, 내분비 및 대사 질환, 기타 질환으로 구분됩니다. 2024년에는 소화기 질환 분야가 시장에서 가장 큰 점유율을 차지했습니다. 이러한 배경에는 장내 세균총과 과민성 대장증후군(IBS), 염증성 장질환(IBD), 궤양성 대장염, 크론병 등의 질환과 밀접한 관련이 있습니다. 이러한 만성질환은 전 세계적으로 유병률이 높고 장기적인 관리가 필요한 경우가 많기 때문에 장내 세균총을 표적으로 하는 대체요법 및 보조요법에 대한 수요가 발생하고 있습니다.
소화기 질환의 발병과 진행에 있어 미생물 불균형의 역할을 뒷받침하는 연구가 증가하고 있으며, 이는 마이크로바이옴 기반 진단 및 치료에 대한 투자 증가를 촉진하고 있습니다. IBD와 IBS를 타겟으로 한 여러 임상 단계의 제품이 개발 중이며, 과학적 관심과 충족되지 않은 환자 니즈를 반영하고 있습니다. 프로바이오틱스, 신바이오틱스 등 소화기 건강을 위한 마이크로바이옴 보충제가 출시되면서 소비자의 접근성이 더욱 확대되어 이 분야의 시장 성장에 기여하고 있습니다.
2024년 북미는 인간 마이크로바이옴 시장에서 가장 큰 점유율을 차지했습니다. 이러한 배경에는 주요 시장 진입 기업의 강력한 존재감, 선진화된 헬스케어 인프라, 마이크로바이옴 연구개발에 대한 많은 투자 등 여러 가지 요인이 있습니다. 이 지역, 특히 미국에서는 마이크로바이옴 과학에 초점을 맞춘 수많은 임상시험과 학술 공동 연구가 진행되고 있습니다. 또한, 미국 FDA가 VOWST와 Rebyota와 같은 마이크로바이옴 기반 치료제를 최초로 승인하여 중요한 규제 선례가 되는 등 규제적 지원도 시장 성장에 기여하고 있습니다. 또한 북미에서는 장 건강과 예방적 웰빙에 대한 인식이 높아지면서 마이크로바이옴 보충제, 특히 프로바이오틱스와 신바이오틱스에 대한 소비자 기반이 크게 성장하고 있습니다. 높은 의료비 지출, 만성질환 유병률 증가, 유리한 상환 정책으로 인해 북미는 세계 인간 마이크로바이옴 시장에서 선도적인 입지를 더욱 공고히 하고 있습니다.
세계의 인간 마이크로바이옴 시장에 대해 조사했으며, 제품별, 유형별, 질환별, 투여 경로별, 서비스별, 최종사용자별, 지역별 동향, 시장 진입 기업 프로파일 등의 정보를 정리하여 전해드립니다.
목차
제1장 소개
제2장 조사 방법
제3장 주요 요약
제4장 주요 인사이트
제5장 시장 개요
- 소개
- 시장 역학
- 고객 비즈니스에 영향을 미치는 동향/혼란
- 가격 분석
- 밸류체인 분석
- 생태계 분석
- 투자와 자금 조달 시나리오
- 기술 분석
- 특허 분석
- 2025-2026년의 주요 회의와 이벤트
- 사례 연구 분석
- 관세 및 규제 분석
- Porter's Five Forces 분석
- 주요 이해관계자와 구입 기준
- 파이프라인 분석
- AI/생성형 AI가 인간 마이크로바이옴 시장에 미치는 영향
- 미국 관세가 인간 마이크로바이옴 시장에 미치는 영향
- 마이크로바이옴 개입별 GLP-1의 부작용 관리
- 인간 마이크로바이옴 시장 전망 동향
제6장 인간 마이크로바이옴 시장(제품별)
- 소개
- 의약품
- 보충제
- 진단
제7장 인간 마이크로바이옴 의약품·보충제 시장(유형별)
- 소개
- 세균 컨소시엄 이식(BCT)/분변 미생물 이식(FMT)
- 생균 제품(LBP)
- 기타
제8장 인간 마이크로바이옴 시장(질환별)
- 소개
- 감염증
- 위장질환
- 내분비 및 대사질환
- 기타
제9장 인간 마이크로바이옴 의약품·보충제 시장(투여 경로별)
- 소개
- 경구 투여
- 직장 투여
제10장 인간 마이크로바이옴 제조 시장(서비스별)
- 소개
- 균주 개발과 최적화
- 발효와 다운스트림 처리
- 배합과 충전/마감
- 기타
제11장 인간 마이크로바이옴 시장(최종사용자별)
- 소개
- 병원·클리닉
- 장기간병 시설
- 기타
제12장 인간 마이크로바이옴 시장(지역별)
- 소개
- 북미
- 북미의 거시경제 전망
- 미국
- 캐나다
- 유럽
- 유럽의 거시경제 전망
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타
- 아시아태평양
- 아시아태평양의 거시경제 전망
- 중국
- 일본
- 인도
- 한국
- 호주
- 기타
- 라틴아메리카
- 라틴아메리카의 거시경제 전망
- 브라질
- 기타
- 중동
- 중동의 거시경제 전망
- GCC 국가
- 기타
- 아프리카
- 성장을 촉진하기 위한 마이크로바이옴에 관한 연구와 대처 증가
- 아프리카의 거시경제 전망
제13장 경쟁 구도
- 소개
- 주요 진출 기업의 전략/강점
- 매출 분석, 2022-2024년
- 시장 점유율 분석, 2024년
- 기업 평가와 재무 지표
- 브랜드/제품 비교
- 기업 평가 매트릭스 : 주요 진출 기업, 2024년
- 기업 평가 매트릭스 : 스타트업/중소기업, 2024년
- 경쟁 시나리오
제14장 기업 개요
- 주요 진출 기업
- INTERNATIONAL FLAVORS & FRAGRANCES INC.
- SEED HEALTH, INC.
- SERES THERAPEUTICS
- FERRING B.V.
- PENDULUM
- OPTIBIOTIX HEALTH PLC
- BIOGAIA
- MAAT PHARMA
- MICROBA
- BIOMEBANK
- BIOHM HEALTH
- ACTIAL FARMACEUTICA SRL
- RESBIOTIC
- INFINANT HEALTH INC.
- EXEGI PHARMA
- FINCH THERAPEUTICS GROUP, INC.
- INFANT BACTERIAL THERAPEUTICS AB
- VIOME LIFESCIENCES
- GENOVA DIAGNOSTICS
- 기타 기업
- AOBIOME
- GUANGZHOU ZHIYI BIOTECHNOLOGY CO., LTD.
- NUBIYOTA
- OXTHERA
- NEXBIOME
- VEDANTA BIOSCIENCES, INC.
- ENTEROME
- APSEN FARMACEUTICA
- METAGEN, INC.
- SNIPR BIOME
- MIKROBIOMIK
- SYNLOGIC
- GENETIC ANALYSIS
- METABIOMICS
- SUN GENOMICS, INC.
제15장 부록
KSM 25.09.04The Human Microbiome market is expected to reach USD 7.09 billion in 2031 from USD 1.40 billion in 2024, at a CAGR of 31.0% during the forecast period.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2031 |
| Base Year | 2024 |
| Forecast Period | 2025-2031 |
| Units Considered | Value (USD billion) |
| Segments | Product, Disease, Drugs & Supplements Type, Drugs & Supplements Route of Administration, End User, Manufacturing Services, and Region |
| Regions covered | North America, Europe, the Asia Pacific, Latin America, the Middle East, and Africa |
Factors such as the collaborative efforts between the microbiome industry and academia for microbiome research and the growing demand for personalized medicine are driving the growth of the human microbiome market. However, complex regulatory policies adversely impact the commercialization of microbiomes, restraining market growth.
The probiotics segment accounted for the largest share of the human microbiome supplements segment in 2024.
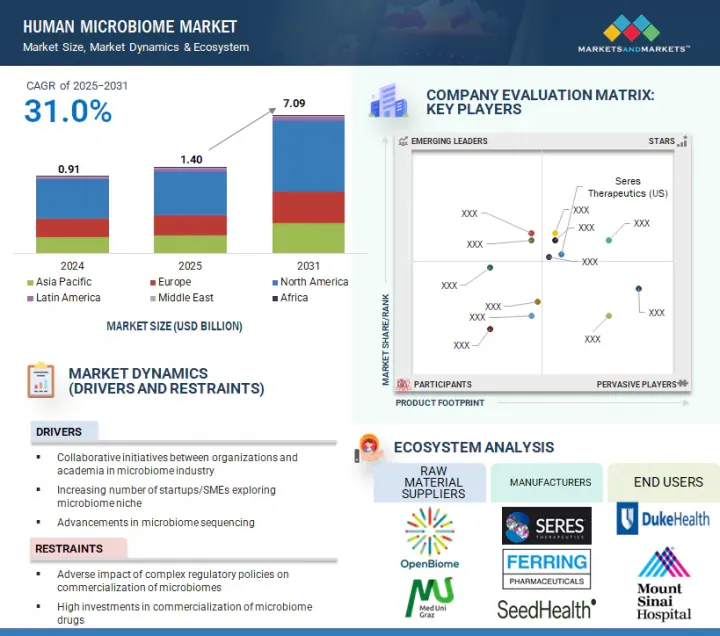
The market is segmented into human microbiome drugs, human microbiome supplements, and human microbiome diagnostics. The human microbiome supplements are analyzed across three key products: probiotics, prebiotics, and synbiotics. The segment with the biggest share in the human microbiome market in 2024 is probiotics. This dominance is attributed to their widespread use, strong consumer awareness, and established presence in over-the-counter health and wellness products. Probiotics are commonly used to support digestive health, boost immunity, and maintain gut microbial balance, making them a routine part of preventive healthcare. They are available in various formats, including capsules, powders, beverages, and functional foods, enhancing accessibility and consumer adoption. Additionally, a large body of clinical research supports the efficacy of specific probiotic strains, reinforcing trust among consumers and healthcare providers. The ability to pair probiotics with other bioactives like prebiotics or vitamins has further expanded their appeal across various demographics. Collectively, these factors have made probiotics the leading product type in the human microbiome supplements segment.
In 2024, the gastrointestinal diseases segment accounted for the largest share of the market.
The human microbiome market is segmented into gastrointestinal diseases, infectious diseases, endocrine & metabolic disorders, and other diseases. In 2024, the gastrointestinal diseases segment accounted for the largest share of the market. This is driven by the strong association between gut microbiota and conditions such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), ulcerative colitis, and Crohn's disease. These chronic disorders have a high global prevalence and often require long-term management, creating demand for alternative and adjunctive therapies that target the gut microbiome.
A growing body of research supports the role of microbial imbalances in the development and progression of gastrointestinal conditions, prompting increased investment in microbiome-based diagnostics and treatments. Several clinical-stage products targeting IBD and IBS are in development, reflecting scientific interest and unmet patient needs. The availability of microbiome supplements aimed at digestive health, such as probiotics and synbiotics, has further expanded consumer access and contributed to market growth in this segment.
In 2024, North America accounted for the largest share of the human microbiome market.
North America accounted for the largest share of the human microbiome market in 2024. This is due to several factors, including a strong presence of key market players, advanced healthcare infrastructure, and high levels of investment in microbiome research and development. The region hosts numerous clinical trials and academic collaborations focused on microbiome science, particularly in the United States. Regulatory support has also contributed to market growth, with the US FDA approving the first microbiome-based therapeutics, such as VOWST and Rebyota, setting important regulatory precedents. In addition, North America has a large and growing consumer base for microbiome supplements, particularly probiotics and synbiotics, driven by rising awareness of gut health and preventive wellness. High healthcare spending, increasing prevalence of chronic diseases, and favorable reimbursement policies further reinforce the leading position of North America in the global human microbiome market.
The primary interviews conducted for this report can be categorized as follows:
- By Respondent: Tier 1-25%, Tier 2-35%, and Tier 3- 40%
- By Designation: C-level Executives - 55%, Directors- 20%, and Others- 25%
- By Region: North America -45%, Europe - 20%, Asia Pacific -20%, Latin America -10%, the Middle East- 3%, and Africa-2%
Seres Therapeutics (US), Ferring B.V. (Switzerland), BiomeBank (Australia), Seed Health, Inc. (US), International Flavors & Fragrances Inc. (US), Pendulum (US), Biohm Technologies (US), Actial Farmaceutica Srl (Italy), OptiBiotix Health Plc (UK), resbiotic (US), Infinant Health Inc (US), BioGaia (Sweden), ExeGi Pharma (US), Finch Therapeutics Group, Inc. (US), Infant Bacterial Therapeutics AB (Sweden), MaaT Pharma (France), Microba (Australia), Viome Life Sciences, Inc. (US), and Genova Diagnostics (US) are some of the key companies offering human microbiome drugs & supplements.
Research Coverage
This research report categorizes the Human Microbiome market by Product (Human Microbiome Drugs, Human Microbiome Supplements [Probiotics, Prebiotics, Synbiotics], Human Microbiome Diagnostics), Disease (Infectious disease, Gastrointestinal Disease, Endocrine, and metabolic disorders, Cancer, and Other Diseases), Drugs & Supplements Type (Bacterial Consortia Transplantation (BCT)/ Fecal Microbiota Transplantation (FMT), Peptides, Live Bacteria Products, and Others), Drugs & Supplements Route of Administration (Oral, Rectal), End User (Hospitals & Clinics, Long-term care Facilities, Other End Users), Manufacturing Service (Strain Development & Optimization, Fermentation & Downstream Processing, Formulation & Fill/Finish, Other Services) and by region (North America, Europe, Asia Pacific, Latin America, Middle East, and Africa). The scope of the report covers detailed information regarding the major factors, such as drivers, challenges, opportunities, and restraints influencing the growth of the Human Microbiome market. A detailed analysis of the key industry players has been done to provide insights into their business overview, service portfolio, key strategies such as collaborations, partnerships, expansions, agreements, and acquisitions, and recent developments associated with the Human Microbiome market. Competitive analysis of top players and upcoming startups in the human microbiome market ecosystem is covered in this report.
The scope of the report covers detailed information regarding the primary factors, such as drivers, restraints, challenges, and opportunities, influencing the growth of the human microbiome market. A thorough analysis of the key industry players has been conducted to provide insights into their business overview, solutions, and services; key strategies; new product & service launches, acquisitions, and recent developments associated with the human microbiome market. This report covers the competitive analysis of upcoming startups in the human microbiome market ecosystem.
Key Benefits of Buying the Report
The report will help market leaders/new entrants by providing them with the closest approximations of the revenue numbers for the overall Human Microbiome market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to better position their business and make suitable go-to-market strategies. This report will enable stakeholders to understand the market's pulse and provide them with information on the key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
Analysis of key drivers (Collaborative initiatives between organizations, academia, and the microbiome industry, Increase in the number of Startups,/SMEs exploring the microbiome niche, Advancements in microbiome sequencing), restraints (Adverse impact of complex regulatory policies on commercialization of microbiomes, High investment in commercialization of microbiome drugs), opportunities (Increase in demand for personalized medicine, and Emergence of synbiotics), and challenges (Slow patient adoption of microbiome-based therapies and Complexities involved in development of microbiome therapies) influencing the growth of the market.
- Product Development/Innovation: Detailed insights on newly launched products of the human microbiome market
- Market Development: Comprehensive information about lucrative markets - the report analyses the market across varied regions.
- Market Diversification: Exhaustive information about new services, untapped geographies, recent developments, and investments in the Human Microbiome market
Competitive Assessment: Seres Therapeutics (US), Ferring B.V. (Switzerland), BiomeBank (Australia), Seed Health, Inc. (US), International Flavors & Fragrances Inc. (US), Pendulum (US), Biohm Technologies (US), Actial Farmaceutica Srl (Italy), OptiBiotix Health Plc (UK), resbiotic (US), Infinant Health Inc (US), BioGaia (Sweden), ExeGi Pharma (US), Finch Therapeutics Group, Inc. (US), Infant Bacterial Therapeutics AB (Sweden), MaaT Pharma (France), Microba (Australia), Viome Life Sciences, Inc. (US), and Genova Diagnostics (US) among others in the market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION AND REGIONAL SCOPE
- 1.3.2 INCLUSIONS AND EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.4 CURRENCY CONSIDERED
- 1.5 STAKEHOLDERS
- 1.6 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.2 PRIMARY DATA
- 2.2 MARKET SIZE ESTIMATION METHODOLOGY
- 2.2.1 INSIGHTS FROM PRIMARY EXPERTS
- 2.2.2 SEGMENTAL MARKET SIZE ESTIMATION
- 2.3 GROWTH RATE PROJECTIONS
- 2.4 MARKET BREAKDOWN AND DATA TRIANGULATION
- 2.5 RESEARCH ASSUMPTIONS
- 2.6 RESEARCH LIMITATIONS
- 2.7 RISK ANALYSIS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 HUMAN MICROBIOME MARKET OVERVIEW
- 4.2 NORTH AMERICA: HUMAN MICROBIOME MARKET, BY TYPE AND COUNTRY, 2024
- 4.3 HUMAN MICROBIOME MARKET, BY TYPE, 2025 VS. 2031
- 4.4 HUMAN MICROBIOME MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- 4.5 HUMAN MICROBIOME MARKET, BY END USER, 2025
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Collaborative initiatives between organizations and academia
- 5.2.1.2 Increasing number of startups/SMEs exploring microbiome
- 5.2.1.3 Advancements in microbiome sequencing
- 5.2.2 RESTRAINTS
- 5.2.2.1 Complex regulatory policies
- 5.2.2.2 High investments in commercializing microbiome drugs
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Increasing demand for personalized medicines
- 5.2.3.2 Emergence of postbiotics
- 5.2.4 CHALLENGES
- 5.2.4.1 Slow patient adoption of microbiome-based therapies
- 5.2.4.2 Complexities in developing microbiome therapies
- 5.2.1 DRIVERS
- 5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 5.3.1 DISRUPTION INDEX: MARKET READINESS VS. ADOPTION VS. MATURITY
- 5.3.1.1 Market readiness
- 5.3.1.2 Adoption
- 5.3.1.3 Maturity
- 5.3.1 DISRUPTION INDEX: MARKET READINESS VS. ADOPTION VS. MATURITY
- 5.4 PRICING ANALYSIS
- 5.4.1 AVERAGE SELLING PRICE TREND OF PRODUCTS, BY KEY PLAYER, 2022-2024
- 5.4.2 AVERAGE SELLING PRICE TREND OF PRODUCTS, BY REGION, 2022-2024
- 5.5 VALUE CHAIN ANALYSIS
- 5.5.1 HUMAN MICROBIOME DRUGS
- 5.5.2 HUMAN MICROBIOME SUPPLEMENTS
- 5.5.3 HUMAN MICROBIOME DIAGNOSTICS
- 5.6 ECOSYSTEM ANALYSIS
- 5.6.1 ECOSYSTEM SHIFT
- 5.6.1.1 Specialized raw material and data suppliers
- 5.6.1.2 Hot-bet innovator startups
- 5.6.1.3 Integrated end users and prosumer models
- 5.6.1.4 Regulatory and quality assurance orchestrators
- 5.6.1.5 Diagnostics-driven personalization
- 5.6.1.6 Microbiome-as-a-Service (Maas)
- 5.6.2 EMERGING BUSINESS MODELS
- 5.6.2.1 Emerging B2C model
- 5.6.2.2 Personalized supplements model
- 5.6.3 INTERCONNECTED MARKET DYNAMICS
- 5.6.1 ECOSYSTEM SHIFT
- 5.7 INVESTMENT AND FUNDING SCENARIO
- 5.7.1 MAJOR INVESTMENTS AND FUNDING BY KEY MARKET PLAYERS
- 5.7.2 OTHER INVESTMENTS AND FUNDING
- 5.8 TECHNOLOGY ANALYSIS
- 5.8.1 KEY TECHNOLOGIES
- 5.8.1.1 Whole-genome sequencing
- 5.8.1.2 16s RNA sequencing method
- 5.8.1.3 Nanopore sequencing
- 5.8.2 COMPLEMENTARY TECHNOLOGIES
- 5.8.2.1 Metatranscriptomics
- 5.8.2.2 Metagenomics
- 5.8.2.3 Metabolomics
- 5.8.3 ADJACENT TECHNOLOGIES
- 5.8.3.1 Sample preparation
- 5.8.3.2 Data analysis
- 5.8.3.3 Library synthesis
- 5.8.1 KEY TECHNOLOGIES
- 5.9 PATENT ANALYSIS
- 5.10 KEY CONFERENCES AND EVENTS, 2025-2026
- 5.11 CASE STUDY ANALYSIS
- 5.11.1 EFFECT OF REBYOTA ON QUALITY OF LIFE IN PATIENTS WITH RECURRENT CLOSTRIDIOIDES DIFFICILE INFECTION
- 5.11.2 ECOSPOR IV STUDY OF VOWST FOR CLOSTRIDIOIDES DIFFICILE INFECTION
- 5.11.3 DELONG#3 STUDY FOR QUALITY OF LIFE IMPROVEMENT BY VSL#3 PROBIOTIC IN POST-COVID-19 PATIENTS
- 5.12 TARIFF AND REGULATORY ANALYSIS
- 5.12.1 TARIFF DATA (HS CODE 3002.90)
- 5.12.2 REGULATORY LANDSCAPE
- 5.12.2.1 North America
- 5.12.2.1.1 US
- 5.12.2.1.2 Canada
- 5.12.2.2 Europe
- 5.12.2.2.1 UK
- 5.12.2.3 Asia Pacific
- 5.12.2.3.1 China
- 5.12.2.3.2 Japan
- 5.12.2.3.3 South Korea
- 5.12.2.3.4 Australia
- 5.12.2.3.5 Rest of Asia Pacific
- 5.12.2.1 North America
- 5.12.3 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.13 PORTER'S FIVE FORCES ANALYSIS
- 5.13.1 BARGAINING POWER OF SUPPLIERS
- 5.13.2 BARGAINING POWER OF BUYERS
- 5.13.3 THREAT OF NEW ENTRANTS
- 5.13.4 THREAT OF SUBSTITUTES
- 5.13.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.14 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.14.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.14.2 BUYING CRITERIA
- 5.15 PIPELINE ANALYSIS
- 5.16 IMPACT OF AI/GEN AI ON HUMAN MICROBIOME MARKET
- 5.16.1 INTRODUCTION
- 5.16.2 MARKET POTENTIAL OF AI IN HUMAN MICROBIOME MARKET
- 5.16.3 AI USE CASES
- 5.16.4 KEY PLAYERS IMPLEMENTING AI
- 5.16.5 FUTURE OF GENERATIVE AI IN HUMAN MICROBIOME MARKET
- 5.17 IMPACT OF US TARIFF ON HUMAN MICROBIOME MARKET
- 5.17.1 KEY TARIFF RATES
- 5.17.2 PRICE IMPACT ANALYSIS
- 5.17.3 KEY IMPACT ON COUNTRY/REGION
- 5.17.3.1 US
- 5.17.3.2 Europe
- 5.17.3.3 Asia Pacific
- 5.17.3.4 Rest of the World
- 5.17.4 IMPACT ON END-USE INDUSTRY
- 5.17.4.1 Hospitals and clinics
- 5.17.4.2 Long-term care facilities
- 5.18 MANAGING GLP-1 SIDE EFFECTS WITH MICROBIOME INTERVENTIONS
- 5.19 FUTURE TRENDS IN HUMAN MICROBIOME MARKET
- 5.19.1 TYPE
- 5.19.2 DISEASE
- 5.19.3 END USER
6 HUMAN MICROBIOME MARKET, BY PRODUCT
- 6.1 INTRODUCTION
- 6.2 DRUGS
- 6.2.1 GROWING PIPELINE OF MICROBIOME-BASED DRUGS IN LATE-STAGE CLINICAL TRIALS TO DRIVE MARKET
- 6.3 SUPPLEMENTS
- 6.3.1 PROBIOTICS
- 6.3.1.1 Increasing consumer awareness about probiotics for improved gut health and immunity to aid growth
- 6.3.2 PREBIOTICS
- 6.3.2.1 Rising development of targeted prebiotics to expedite growth
- 6.3.3 SYNBIOTICS
- 6.3.3.1 Growing demand for personalized treatment and rising incidence of dysbiosis to fuel market
- 6.3.1 PROBIOTICS
- 6.4 DIAGNOSTICS
- 6.4.1 INCREASING PRODUCT LAUNCHES AND TECHNOLOGY TRANSFER TO FACILITATE GROWTH
7 HUMAN MICROBIOME DRUGS & SUPPLEMENTS MARKET, BY TYPE
- 7.1 INTRODUCTION
- 7.2 BACTERIAL CONSORTIA TRANSPLANTATION (BCT)/FECAL MICROBIOTA TRANSPLANTATION (FMT)
- 7.2.1 ADVANCEMENTS IN CLINICAL TRIALS FOR TREATMENT OF C. DIFFICILE INFECTION TO AUGMENT GROWTH
- 7.3 LIVE BACTERIA PRODUCTS (LBP)
- 7.3.1 INCREASING CONSUMER FOCUS ON PREVENTIVE HEALTH TO AID GROWTH
- 7.4 OTHER MICROBIOME TYPES
8 HUMAN MICROBIOME MARKET, BY DISEASE
- 8.1 INTRODUCTION
- 8.2 INFECTIOUS DISEASES
- 8.2.1 EMERGENCE OF MULTIDRUG-RESISTANT BACTERIA TO FOSTER GROWTH
- 8.3 GASTROINTESTINAL DISEASES
- 8.3.1 INCREASING DEMAND FOR PERSONALIZED THERAPIES TO AID GROWTH
- 8.4 ENDOCRINE & METABOLIC DISORDERS
- 8.4.1 DEVELOPMENT OF MICROBIOME MODULATORS FOR METABOLIC DISORDERS TO BOOST MARKET
- 8.5 OTHER DISEASES
9 HUMAN MICROBIOME DRUGS & SUPPLEMENTS MARKET, BY ROUTE OF ADMINISTRATION
- 9.1 INTRODUCTION
- 9.2 ORAL ROUTE OF ADMINISTRATION
- 9.2.1 WIDESPREAD PATIENT ACCEPTANCE AND EXTENSIVE PRODUCT OFFERINGS TO ENCOURAGE GROWTH
- 9.3 RECTAL ROUTE OF ADMINISTRATION
- 9.3.1 ABILITY TO RESTORE HEALTHY GUT TO FACILITATE GROWTH
10 HUMAN MICROBIOME MANUFACTURING MARKET, BY SERVICE
- 10.1 INTRODUCTION
- 10.2 STRAIN DEVELOPMENT & OPTIMIZATION
- 10.2.1 RISING DEMAND FOR TARGETED, STRAIN-SPECIFIC MICROBIOME THERAPIES TO BOOST MARKET
- 10.3 FERMENTATION & DOWNSTREAM PROCESSING
- 10.3.1 GROWING DEMAND FOR CLINICAL-STAGE BIOTHERAPEUTICS TO DRIVE MARKET
- 10.4 FORMULATION & FILL/FINISH
- 10.4.1 INCREASING STUDIES ON LIVE BIOTHERAPEUTIC PRODUCTS TO PROMOTE GROWTH
- 10.5 OTHER SERVICES
11 HUMAN MICROBIOME MARKET, BY END USER
- 11.1 INTRODUCTION
- 11.2 HOSPITALS & CLINICS
- 11.2.1 AVAILABILITY OF ADVANCED HEALTHCARE INFRASTRUCTURE AND EXPERTISE TO AUGMENT GROWTH
- 11.3 LONG-TERM CARE FACILITIES
- 11.3.1 NEED FOR SPECIALIZED CARE AND CONTINUOUS MONITORING FOR PATIENTS WITH CHRONIC HEALTH CONDITIONS TO DRIVE MARKET
- 11.4 OTHER END USERS
12 HUMAN MICROBIOME MARKET, BY REGION
- 12.1 INTRODUCTION
- 12.2 NORTH AMERICA
- 12.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA
- 12.2.2 US
- 12.2.2.1 Favorable government initiatives and booming diagnostics sector to drive market
- 12.2.3 CANADA
- 12.2.3.1 Increasing prevalence of chronic diseases to expedite growth
- 12.3 EUROPE
- 12.3.1 MACROECONOMIC OUTLOOK FOR EUROPE
- 12.3.2 GERMANY
- 12.3.2.1 Rapid increase in government and private funding to propel market
- 12.3.3 UK
- 12.3.3.1 Significant government investments in microbiome research and presence of microbiome companies to propel growth
- 12.3.4 FRANCE
- 12.3.4.1 Growing focus on developing new products and services using microbiome data to boost market
- 12.3.5 ITALY
- 12.3.5.1 Rise in research activities to encourage growth
- 12.3.6 SPAIN
- 12.3.6.1 Favorable microbiome research landscape to support growth
- 12.3.7 REST OF EUROPE
- 12.4 ASIA PACIFIC
- 12.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
- 12.4.2 CHINA
- 12.4.2.1 Rapidly expanding healthcare industry to accelerate growth
- 12.4.3 JAPAN
- 12.4.3.1 Booming geriatric population to contribute to growth
- 12.4.4 INDIA
- 12.4.4.1 Increasing number of human microbiome diagnostic companies to augment growth
- 12.4.5 SOUTH KOREA
- 12.4.5.1 Booming bio-health market to advance growth
- 12.4.6 AUSTRALIA
- 12.4.6.1 Growing number of product approvals to boost markt
- 12.4.7 REST OF ASIA PACIFIC
- 12.5 LATIN AMERICA
- 12.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA
- 12.5.2 BRAZIL
- 12.5.2.1 Rising probiotics demand to drive market
- 12.5.3 REST OF LATIN AMERICA
- 12.6 MIDDLE EAST
- 12.6.1 MACROECONOMIC OUTLOOK IN MIDDLE EAST
- 12.6.2 GCC COUNTRIES
- 12.6.2.1 Saudi Arabia
- 12.6.2.1.1 Growing focus on healthcare infrastructure to fuel market
- 12.6.2.2 UAE
- 12.6.2.2.1 Increasing focus on immunotherapy and biotechnology to augment growth
- 12.6.2.3 Rest of GCC countries
- 12.6.2.1 Saudi Arabia
- 12.6.3 REST OF MIDDLE EAST
- 12.7 AFRICA
- 12.7.1 INCREASING STUDIES AND INITIATIVES ON MICROBIOME TO PROMOTE GROWTH
- 12.7.2 MACROECONOMIC OUTLOOK FOR AFRICA
13 COMPETITIVE LANDSCAPE
- 13.1 INTRODUCTION
- 13.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 13.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN HUMAN MICROBIOME MARKET
- 13.3 REVENUE ANALYSIS, 2022-2024
- 13.4 MARKET SHARE ANALYSIS, 2024
- 13.5 COMPANY VALUATION AND FINANCIAL METRICS
- 13.6 BRAND/PRODUCT COMPARISON
- 13.7 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 13.7.1 STARS
- 13.7.2 EMERGING LEADERS
- 13.7.3 PERVASIVE PLAYERS
- 13.7.4 PARTICIPANTS
- 13.7.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 13.7.5.1 Company footprint
- 13.7.5.2 Region footprint
- 13.7.5.3 Product footprint
- 13.7.5.4 Type footprint
- 13.7.5.5 Disease footprint
- 13.8 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 13.8.1 PROGRESSIVE COMPANIES
- 13.8.2 RESPONSIVE COMPANIES
- 13.8.3 DYNAMIC COMPANIES
- 13.8.4 STARTING BLOCKS
- 13.8.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 13.8.5.1 Detailed list of key startups/SMEs
- 13.8.5.2 Competitive benchmarking of key emerging players/startups
- 13.9 COMPETITIVE SCENARIO
- 13.9.1 PRODUCT LAUNCHES AND APPROVALS
- 13.9.2 DEALS
- 13.9.3 EXPANSIONS
14 COMPANY PROFILES
- 14.1 KEY PLAYERS
- 14.1.1 INTERNATIONAL FLAVORS & FRAGRANCES INC.
- 14.1.1.1 Business overview
- 14.1.1.2 Products offered
- 14.1.1.3 Recent developments
- 14.1.1.3.1 Deals
- 14.1.1.3.2 Expansions
- 14.1.1.4 MnM view
- 14.1.1.4.1 Key strengths
- 14.1.1.4.2 Strategic choices
- 14.1.1.4.3 Weaknesses and competitive threats
- 14.1.2 SEED HEALTH, INC.
- 14.1.2.1 Business overview
- 14.1.2.2 Products offered
- 14.1.2.3 Recent developments
- 14.1.2.3.1 Product launches and approvals
- 14.1.2.3.2 Deals
- 14.1.2.3.3 Expansions
- 14.1.2.4 MnM view
- 14.1.2.4.1 Key strengths
- 14.1.2.4.2 Strategic choices
- 14.1.2.4.3 Weaknesses and competitive threats
- 14.1.3 SERES THERAPEUTICS
- 14.1.3.1 Business overview
- 14.1.3.2 Products offered
- 14.1.3.3 Recent developments
- 14.1.3.3.1 Product launches and approvals
- 14.1.3.3.2 Deals
- 14.1.3.4 MnM view
- 14.1.3.4.1 Key strengths
- 14.1.3.4.2 Strategic choices
- 14.1.3.4.3 Weaknesses and competitive threats
- 14.1.4 FERRING B.V.
- 14.1.4.1 Business overview
- 14.1.4.2 Products offered
- 14.1.4.3 Recent developments
- 14.1.4.3.1 Product launches and approvals
- 14.1.4.3.2 Deals
- 14.1.4.4 MnM view
- 14.1.4.4.1 Key strengths
- 14.1.4.4.2 Strategic choices
- 14.1.4.4.3 Weaknesses and competitive threats
- 14.1.5 PENDULUM
- 14.1.5.1 Business overview
- 14.1.5.2 Products offered
- 14.1.5.3 Recent developments
- 14.1.5.3.1 Product launches and approvals
- 14.1.5.3.2 Deals
- 14.1.5.4 MnM view
- 14.1.5.4.1 Key strengths
- 14.1.5.4.2 Strategic choices
- 14.1.5.4.3 Weaknesses and competitive threats
- 14.1.6 OPTIBIOTIX HEALTH PLC
- 14.1.6.1 Business overview
- 14.1.6.2 Products offered
- 14.1.6.3 Recent developments
- 14.1.6.3.1 Product launches and approvals
- 14.1.6.3.2 Deals
- 14.1.7 BIOGAIA
- 14.1.7.1 Business overview
- 14.1.7.2 Products offered
- 14.1.7.3 Recent developments
- 14.1.7.3.1 Product launches and approvals
- 14.1.7.3.2 Deals
- 14.1.7.3.3 Expansions
- 14.1.8 MAAT PHARMA
- 14.1.8.1 Business overview
- 14.1.8.2 Products offered
- 14.1.8.3 Recent developments
- 14.1.8.3.1 Deals
- 14.1.8.3.2 Other developments
- 14.1.9 MICROBA
- 14.1.9.1 Business overview
- 14.1.9.2 Products offered
- 14.1.9.3 Recent developments
- 14.1.9.3.1 Product launches and approvals
- 14.1.10 BIOMEBANK
- 14.1.10.1 Business overview
- 14.1.10.2 Products offered
- 14.1.10.3 Recent developments
- 14.1.10.3.1 Product launches and approvals
- 14.1.10.3.2 Deals
- 14.1.10.3.3 Expansions
- 14.1.11 BIOHM HEALTH
- 14.1.11.1 Business overview
- 14.1.11.2 Products offered
- 14.1.11.3 Recent developments
- 14.1.11.3.1 Deals
- 14.1.12 ACTIAL FARMACEUTICA SRL
- 14.1.12.1 Business overview
- 14.1.12.2 Products offered
- 14.1.13 RESBIOTIC
- 14.1.13.1 Business overview
- 14.1.13.2 Products offered
- 14.1.13.3 Recent developments
- 14.1.13.3.1 Product launches and approvals
- 14.1.13.3.2 Deals
- 14.1.13.3.3 Expansions
- 14.1.14 INFINANT HEALTH INC.
- 14.1.14.1 Business overview
- 14.1.14.2 Products offered
- 14.1.14.3 Recent developments
- 14.1.14.3.1 Deals
- 14.1.15 EXEGI PHARMA
- 14.1.15.1 Business overview
- 14.1.15.2 Products offered
- 14.1.15.3 Recent developments
- 14.1.15.3.1 Deals
- 14.1.16 FINCH THERAPEUTICS GROUP, INC.
- 14.1.16.1 Business overview
- 14.1.16.2 Products offered
- 14.1.16.3 Recent developments
- 14.1.16.3.1 Deals
- 14.1.17 INFANT BACTERIAL THERAPEUTICS AB
- 14.1.17.1 Business overview
- 14.1.17.2 Products offered
- 14.1.18 VIOME LIFESCIENCES
- 14.1.18.1 Business overview
- 14.1.18.2 Products offered
- 14.1.18.3 Recent developments
- 14.1.18.3.1 Product launches and approvals
- 14.1.19 GENOVA DIAGNOSTICS
- 14.1.19.1 Business overview
- 14.1.19.2 Products offered
- 14.1.1 INTERNATIONAL FLAVORS & FRAGRANCES INC.
- 14.2 OTHER PLAYERS
- 14.2.1 AOBIOME
- 14.2.2 GUANGZHOU ZHIYI BIOTECHNOLOGY CO., LTD.
- 14.2.3 NUBIYOTA
- 14.2.4 OXTHERA
- 14.2.5 NEXBIOME
- 14.2.6 VEDANTA BIOSCIENCES, INC.
- 14.2.7 ENTEROME
- 14.2.8 APSEN FARMACEUTICA
- 14.2.9 METAGEN, INC.
- 14.2.10 SNIPR BIOME
- 14.2.11 MIKROBIOMIK
- 14.2.12 SYNLOGIC
- 14.2.13 GENETIC ANALYSIS
- 14.2.14 METABIOMICS
- 14.2.15 SUN GENOMICS, INC.
15 APPENDIX
- 15.1 DISCUSSION GUIDE
- 15.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 15.3 CUSTOMIZATION OPTIONS
- 15.4 RELATED REPORTS
- 15.5 AUTHOR DETAILS



















