
|
시장보고서
상품코드
1801772
표적 단백질 분해 시장 : 유형별, 적응증별, 제형별, 최종 사용자별 예측(-2035년)Targeted Protein Degradation Market by Type [PROTACs (Vepdegestrant, Bavdegalutamide), SERDs (Elacestrant), Molecular Glues (Mezigdomide), LDD, LYTAC/ATAC], Indication (Oncology, Inflammatory), Formulation (Oral), End User - Global Forecast to 2035 |
||||||
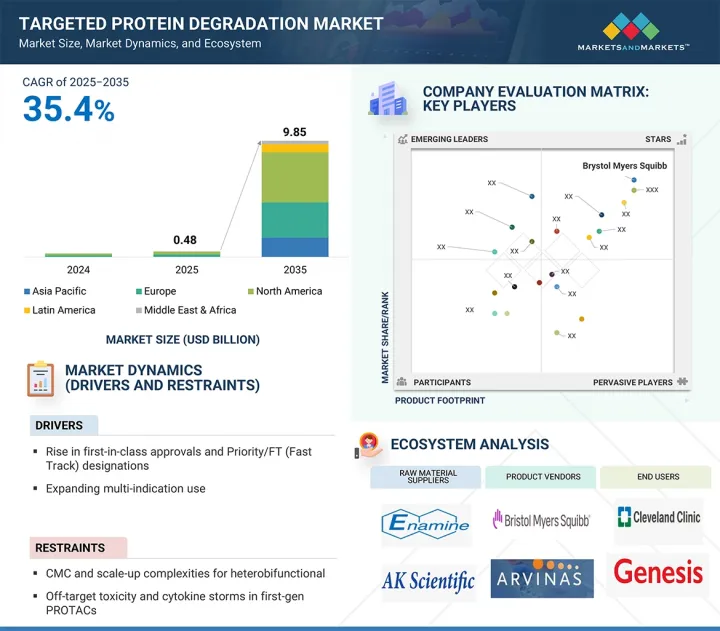
세계의 표적 단백질 분해 시장 규모는 2025년 추정 4억 8,000만 달러에서 2035년까지 98억 5,000만 달러에 이를 것으로 예측되며, 2025-2035년 CAGR은 35.4%를 나타낼 전망입니다.
| 조사 범위 | |
|---|---|
| 조사 대상 연도 | 2024-2035년 |
| 기준 연도 | 2024년 |
| 예측 기간 | 2025-2035년 |
| 단위 | 10억 달러 |
| 부문 | 유형, 치료 적응증, 제형, 최종 사용자, 지역 |
| 대상 지역 | 북미, 유럽, 아시아태평양, 라틴아메리카, 중동 및 아프리카 |
표적 단백질 분해 시장 확대는 주로 자본 유입, 대형 제약사 제휴, 그리고 다양한 적응증 확대에 의해 촉진되어 왔습니다. 그러나 CMC(핵산·단백질·단백질 분해) 및 이종이식 의약품(Heterobifunctional)과 지적재산권(IP) 분쟁으로 인한 규모 확장의 복잡성은 시장 성장을 억제할 것으로 예측됩니다.
치료 적응증 부문에서는 암 부문이 2024년에 가장 높은 CAGR을 나타냈습니다.
암 부문은 모든 치료 적응증 부문 중에서 표적 단백질 분해 시장에서 가장 높은 CAGR을 나타낼 것으로 예측됩니다. 이 급성장은 세계의 암 이환율 증가, 과거에는 치료 불가능하다고 여겨지고 있던 질환의 원인이 되는 단백질을 표적으로 하는 치료법에 대한 긴급의 요구에 의한 것입니다. PROTACs와 분자 접착제를 포함한 TPD 기술은 중요한 발암성 단백질을 억제하는 대신 분해함으로써 보다 완전하고 지속적인 치료 효과를 제공하는 획기적인 접근법을 제공합니다. 여러 바이오테크놀러지 기업과 제약 기업이 전립선암, 유방암, 폐암, 혈액암, 고형암 등 암을 적응증으로 한 TPD 후보약의 개발을 진행하고 있습니다. Arvinas, Kymera Therapeutics, Nurix Therapeutics, C4 Therapeutics 등의 업계 리더들은 AR, ER, STAT3, BTK 등 중요한 암 관련 단백질을 표적으로 하는 임상 단계 분해제를 개발하고 있습니다. 또한 주요 제약 기업과의 파트너십과 규제 당국의 지원 증가로 개발 일정이 가속화되고 있습니다.
정밀의료에 대한 주목의 높아짐, 암 이환율의 상승, 기존 요법의 한계에 의해 암부문은 표적 단백질 분해 시장을 독점할 전망입니다. TPD 설계, 바이오마커 통합, 병용요법에 있어서의 지속적인 기술 혁신이 성장을 더욱 촉진하고, 이 시장에서 가장 역동적이고 유망한 응용 분야로서의 암의 역할을 확고한 것으로 보입니다.
최종 사용자별로, 가정 관리 환경 부문은 표적 단백질 분해 시장에서 가장 높은 CAGR을 나타냈습니다.
2024년, 환자 중심의 편리하고 비용 효율적인 치료 옵션에 대한 수요가 증가함에 따라, 재택 케어 환경 부문이 표적 단백질 분해 시장에서 가장 빠르게 성장하는 최종 사용자 부문으로 부상했습니다. 이러한 추세에 기여하는 주요 요인 중 하나는 경구 TPD 제형의 개발의 진행이며, 이는 환자가 집에 있을 때 암이나 자가면역 질환과 같은 복잡한 병리학을 관리할 수 있습니다. 병원에서 투여해야 하는 기존의 생물학적 제형과는 달리, 경구분해제는 빈번한 통원을 필요로 하지 않으며 안전하고 효과적인 치료를 가능하게 하며 환자의 컴플라이언스와 QOL을 크게 향상시킵니다. 재택 치료로의 전환은 원격 환자 모니터링 및 원격 의료 플랫폼과 같은 디지털 건강 기술의 통합을 통해 더욱 향상되고, 의사는 실시간으로 치료 반응을 추적하고 부작용을 관리 할 수 있습니다. 의료 시스템은 병원 부담을 줄이고 치료비를 억제하기 위해 재택 관리 모델을 적극적으로 장려합니다. 많은 표적 단백질 분해제가 후기 임상개발을 거쳐 규제 당국의 승인을 얻고 있기 때문에 재택 케어의 우위성은 강해질 것으로 예측됩니다. 편의성, 확장성, 현대 의료 제공 모델과의 일관성으로 인해, 가정 관리 환경은 진화하는 치료 상황에서 TPD 치료의 중요하고 확장되는 채널이 됩니다.
북미가 2025-2030년 세계의 표적 단백질 분해 시장에서 가장 큰 점유율을 차지할 전망입니다.
북미는 생명공학 혁신의 탄탄한 기반, 유리한 규제 상황, 활발한 투자 활동을 통해 표적 단백질 분해 시장에서 가장 큰 점유율을 차지했습니다. 이 지역에는 Arvinas, Kymera Therapeutics, Nurix Therapeutics, C4 Therapeutics 등 최첨단 분해 기술을 임상 개발로 추진하는 선구적인 TPD 기업이 있습니다. 또한 Bristol Myers Squibb 및 Pfizer와 같은 주요 제약 회사는 공동 연구 및 사내 연구 개발을 통해 TPD 파이프라인을 적극적으로 확대하고 있습니다. 이 지역은 성숙한 의료 인프라, 최상급 학술 연구 기관에 대한 접근, 신규 치료법의 조기 채용으로부터 혜택을 누리고 있습니다.
이 보고서는 세계의 표적 단백질 분해 시장에 대한 조사 분석을 통해 주요 성장 촉진요인과 억제요인, 경쟁 구도, 미래 동향 등의 정보를 제공합니다.
목차
제1장 서론
제2장 조사 방법
제3장 주요 요약
제4장 중요한 지견
- 표적 단백질 분해 시장 개요
- 북미의 표적 단백질 분해 시장 : 분해약 유형별, 국가별(2030년)
- 표적 단백질 분해 시장 : 지리적 성장 기회
- 표적 단백질 분해 시장 : 신흥 시장 vs. 선진 시장
제5장 시장 개요
- 서론
- 시장 역학
- 성장 촉진요인
- 성장 억제요인
- 기회
- 과제
- 기술 분석
- 주요 기술
- 보완 기술
- 인접 기술
- 고객사업에 영향을 주는 동향/혼란
- 가격 설정 분석
- ELACESTRANT(ORSERDU)의 가격 설정에 관한 정성적인 인사이트(2024년)
- 향후 신규 표적 단백질 분해제의 가격 설정에 관한 정성적인 인사이트
- ELACESTRANT(ORSERDU)의 상환 시나리오
- 미국
- 유럽
- 밸류체인 분석
- 생태계 분석
- 특허 분석
- 파이프라인 분석
- 규제 분석
- 지역별 규제기관, 정부기관, 기타 조직
- 규제 틀
- 주요 컨퍼런스 및 이벤트(2025-2026년)
- Porter's Five Forces 분석
- 주요 이해관계자와 구매 기준
- 투자 및 자금조달 시나리오
- 표적 단백질 분해 시장에 대한 AI/생성형 AI의 영향
- 표적 단백질 분해 시장에 대한 미국 관세의 영향(2025년)
- 서론
- 주요 관세율
- 가격의 영향 분석
- 국가/지역에 미치는 영향
- 최종 이용 산업에 미치는 영향
제6장 표적 단백질 분해 시장 : 분해약 유형별
- 서론
- 분자 접착
- MEZIGDOMIDE(CC-92480)
- IBERDOMIDE(CC-220)
- SERD
- ELACESTRANT
- GIREDESTRANT(GDC9545)
- CAMIZESTRANT(AZD9833)
- PROTAC
- VEPDEGESTRANT(ARV-471)
- LUXDEGALUTAMIDE
- BGB-16673
- NX-5948
- KT-474
- LDD/BIDAC
- LYTAC/ATAC
- AUTAC/ATTEC
제7장 표적 단백질 분해 시장 : 치료 적응증별
- 서론
- 종양학
- 염증성 질환
- 기타 질환
제8장 표적 단백질 분해 시장 : 제형별
- 서론
- 경구 제형
- 정제
- 캡슐
- 주사제
제9장 표적 단백질 분해 시장 : 최종 사용자별
- 서론
- 병원 및 전문 클리닉
- 장기 요양 시설
- 재택 간호 환경
제10장 표적 단백질 분해 시장 : 지역별
- 서론
- 북미
- 북미의 거시경제 분석
- 미국
- 캐나다
- 유럽
- 유럽의 거시경제 분석
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 아시아태평양의 거시경제 분석
- 중국
- 일본
- 인도
- 한국
- 호주
- 기타 아시아태평양
- 라틴아메리카
- 라틴아메리카의 거시 경제 분석
- 브라질
- 멕시코
- 기타 라틴아메리카
- 중동 및 아프리카
- 중동 및 아프리카의 거시경제 분석
- GCC 국가
- 기타 중동 및 아프리카
제11장 경쟁 구도
- 서론
- 주요 진입기업의 전략/강점
- 수익 분석(2028-2030년)
- 시장 점유율 분석(2030년)
- 기업 평가 및 재무 지표
- 브랜드/제품 비교
- 기업 평가 매트릭스 : 주요 기업(2024년)
- 기업의 평가 매트릭스 : 스타트업/중소기업(2024년)
- 경쟁 시나리오
제12장 기업 프로파일
- 주요 기업
- BRYSTOL-MYERS SQUIBB COMPANY
- THE MENARINI GROUP
- ARVINAS
- BEONE MEDICINES
- NURIX THERAPEUTICS, INC.
- KYMERA THERAPEUTICS, INC.
- C4 THERAPEUTICS, INC.
- ASTRAZENECA
- F. HOFFMANN-LA ROCHE LTD
- BAYER AG
- CAPTOR THERAPEUTICS
- RANOK THERAPEUTICS CO. LTD.
- PFIZER INC.
- NOVARTIS AG
- FOGHORN THERAPEUTICS
- 기타 기업
- MONTE ROSA THERAPEUTICS
- BIOTHERYX, INC.
- CULLGEN
- NEOMORPH
- LYCIA THERAPEUTICS
- PHOTYS THERAPEUTICS
- PLEXIUM, INC.
- SEED THERAPEUTICS, INC.
- AVILAR THERAPEUTICS, INC.
- AUTOMERA
제13장 부록
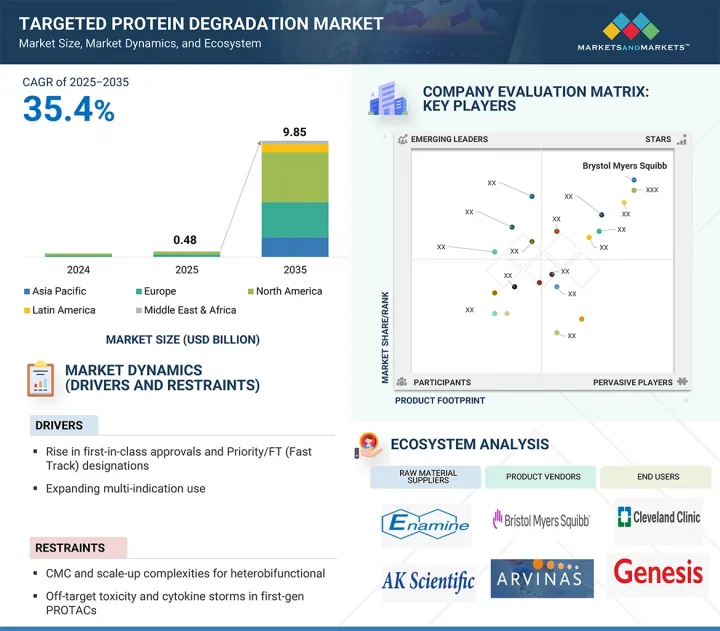
KTH 25.09.11The global targeted protein degradation market is projected to reach USD 9.85 billion by 2035 from an estimated USD 0.48 billion in 2025, at a CAGR of 35.4% from 2025 to 2035.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2035 |
| Base Year | 2024 |
| Forecast Period | 2025-2035 |
| Units Considered | Value (USD billion) |
| Segments | By Type, Therapeutic Indication, Formulation, End User, and Region |
| Regions covered | North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
The expansion of the targeted protein degradation market has been predominantly fueled by Capital inflows & big-pharma tie-ups, and multi-indication expansion. However, CMC & scale-up complexity for heterobifunctional and IP disputes are expected to restrain market growth.
The oncology segment reported the highest CAGR in the therapeutic indications segment in 2024.
Based on therapeutic indications, the market is categorized into oncology, inflammatory diseases, and other therapeutic indications. Oncology is projected to exhibit the highest CAGR in the targeted protein degradation Market among all therapeutic indication segments. This rapid growth is driven by the increasing global burden of cancer and the urgent need for therapies that can target disease-causing proteins once considered undruggable. TPD technologies, including PROTACs and molecular glue degraders, offer a revolutionary approach by degrading, rather than inhibiting key oncogenic proteins, leading to more complete and durable therapeutic responses. Several biotech and pharmaceutical companies are advancing TPD candidates specifically for oncology indications, including prostate, breast, lung, hematologic, and solid tumors. Industry leaders, such as Arvinas, Kymera Therapeutics, Nurix Therapeutics, and C4 Therapeutics, are developing clinical-stage degraders targeting critical cancer-related proteins like AR, ER, STAT3, and BTK. Additionally, big pharma partnerships and increasing regulatory support are accelerating development timelines.
With a growing focus on precision medicine, rising cancer incidence, and limitations of existing therapies, the oncology segment is poised to dominate the targeted protein degradation market. Continued innovation in TPD design, biomarker integration, and combination therapies will further drive growth and solidify oncology's role as the most dynamic and promising application area in this market.
The homecare settings segment registered the highest CAGR in the targeted protein degradation market by end user.
The targeted protein degradation market is segmented by end users into hospitals & specialty clinics, long-term care facilities, and home care settings. In 2024, the home care settings segment emerged as the fastest-growing end-user segment in the targeted protein degraders (TPD) market, driven by the growing demand for patient-centric, convenient, and cost-effective treatment options. One of the key factors contributing to this trend is the increasing development of oral TPD formulations, which allow patients to manage complex conditions like cancer and autoimmune diseases from the comfort of their homes. Unlike traditional biologics requiring hospital administration, oral degraders enable safe, effective treatment without the need for frequent clinic visits, significantly improving patient compliance and quality of life. The shift toward home-based care is further supported by the integration of digital health technologies, including remote patient monitoring and telehealth platforms, which allow physicians to track treatment response and manage side effects in real time. Healthcare systems are actively encouraging homecare models to reduce hospital burden and control treatment costs. As more targeted protein degraders progress through late-stage clinical development and gain regulatory approval, the dominance of home care settings is expected to strengthen. With their convenience, scalability, and alignment with modern healthcare delivery models, home care environments represent a vital and expanding channel for TPD therapies in the evolving treatment landscape.
North America accounted for the largest share in the global targeted protein degradation market from 2025 to 2030.
North America accounted for the largest share in the targeted protein degradation market, driven by a strong foundation in biotechnology innovation, a favorable regulatory landscape, and significant investment activity. The region is home to several pioneering TPD companies, including Arvinas, Kymera Therapeutics, Nurix Therapeutics, and C4 Therapeutics, which are advancing cutting-edge degrader technologies into clinical development. In addition, major pharmaceutical firms such as Bristol Myers Squibb and Pfizer are actively expanding their TPD pipelines through collaborations and internal R&D. The region benefits from a mature healthcare infrastructure, access to top-tier academic research institutions, and early adoption of novel therapeutic modalities. The US Food and Drug Administration (FDA) has shown increasing recognition of TPD-based therapies, granting designations that facilitate faster development and approval. Furthermore, the growing emphasis on oral and home-based treatments aligns well with healthcare delivery models in North America.
These factors collectively position North America as a key driver of innovation, commercialization, and clinical advancement in the global TPD market, making it the fastest-growing regional segment.
The primary interviews conducted for this report can be categorized as follows:
- By Respondent: Supply Side- 70% and Demand Side- 30%
- By Designation: Managers- 45%, CXO and Directors- 30%, and Executives- 25%
- By Region: North America- 30%, Europe- 30%, Asia Pacific- 30%, Latin America- 5%, and the Middle East & Africa- 5%
Key Companies
Key players in the targeted protein degradation market include Bristol Myers Squibb (US), Arvinas (US), BeiGene (US), Nurix (US), Kymera (US), C4 Therapeutics (US), Stemline Therapeutics (US), AstraZeneca (UK), F. Hoffmann-La Roche Ltd (Switzerland), Bayer (Vividion) (Germany), Captor Therapeutics (Poland), Ranok Therapeutics (US), Pfizer (US), Novartis (Switzerland), and Foghorn Therapeutics (US).
Research Coverage
This research report categorizes the targeted protein degradation market, by type [molecular glue (mezigdomide, Iberdomide), SERDs (Elacestrant, Giredestrant, Camizestrant), PROTAC (Vepdegestrant, Bavdegalutamide, BGB-16673, NX-5948, KT-474), LDD/BiDAC, LYTAC/ATAC, Autophhagy-targeting chimeras] therapeutic indication (oncology, inflammatory diseases, and others), Formulation (oral formulationsand injections), end user (hospitals & speciality clinics, long-term care facility and home care settings) and region (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa).
The scope of the report covers detailed information regarding the major factors, such as drivers, restraints, challenges, and opportunities, influencing the growth of the targeted protein degradation market. A detailed analysis of the key industry players has been done to provide insights into their business overview, products, solutions, key strategies, collaborations, partnerships, and agreements. New approvals/launches, collaborations, acquisitions, and recent developments associated with the targeted protein degradation market.
Reasons to buy this report
The report will help market leaders and new entrants by providing them with the closest approximations of the revenue numbers for the overall targeted protein degradation market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to better position their businesses and make suitable go-to-market strategies. This report will enable stakeholders to understand the market's pulse and provide them with information on the key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
- Analysis of key drivers (Increasing first-In-class approvals with priority/FT designations, expanding multi-indication use, increasing capital inflows and big-pharma collaborations, and technological advancements in degrader design and discovery) restraints (CMC and scale-up complexity for heterobifunctionals, off-target toxicity and cytokine storms in first-gen PROTACs, and IP disputes), opportunities (Growing adoption of CNS and immunology degraders in clinics, development of next-gen ligases with tissue-selective expression, and increasing NDA filing for innovative degraders), and challenges (Limited ligase expression and strict regulatory guidelines) influencing the growth of the market.
- Service Development/Innovation: Detailed insights on upcoming technologies, research & development activities in the targeted protein degradation market
- Market Development: Comprehensive information about lucrative markets across varied regions
- Market Diversification: Exhaustive information about untapped geographies, recent developments, and investments in the targeted protein degradation market
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and product offerings of leading players. A detailed analysis of the key industry players has been done to provide insights into their key strategies, product launches/ approvals, acquisitions, partnerships, agreements, collaborations, other recent developments, investment and funding activities, brand/product comparative analysis, and vendor valuation and financial metrics of the targeted protein degradation market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION AND REGIONAL SCOPE
- 1.3.2 INCLUSIONS AND EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.4 CURRENCY CONSIDERED
- 1.5 STAKEHOLDERS
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Objectives of secondary research
- 2.1.1.2 Key data from secondary sources
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Breakdown of primaries (supply- and demand-side participants)
- 2.1.2.2 Key objectives of primary research
- 2.1.1 SECONDARY DATA
- 2.2 MARKET SIZE ESTIMATION
- 2.2.1 GLOBAL MARKET SIZE ESTIMATION
- 2.2.1.1 Company revenue analysis (Bottom-up approach)
- 2.2.1.2 Revenue share analysis
- 2.2.1.3 MnM repository analysis
- 2.2.1.4 Primary interviews
- 2.2.2 INSIGHTS FROM PRIMARY EXPERTS
- 2.2.3 SEGMENTAL MARKET SIZE ESTIMATION (TOP-DOWN APPROACH)
- 2.2.1 GLOBAL MARKET SIZE ESTIMATION
- 2.3 GROWTH RATE PROJECTIONS
- 2.4 DATA TRIANGULATION
- 2.5 RESEARCH ASSUMPTIONS
- 2.6 RESEARCH LIMITATIONS
- 2.7 RISK ANALYSIS
3 EXECUTIVE SUMMARY
- 3.1 STRATEGIC IMPERATIVES FOR KEY STAKEHOLDERS
- 3.1.1 BIOTECH STARTUPS AND INNOVATIVE COMPANIES
- 3.1.2 ESTABLISHED MARKET LEADERS
- 3.1.3 CDMOS AND CROS
4 PREMIUM INSIGHTS
- 4.1 TARGETED PROTEIN DEGRADERS MARKET OVERVIEW
- 4.2 NORTH AMERICA: TARGETED PROTEIN DEGRADERS MARKET, BY DEGRADER TYPE AND COUNTRY, 2030
- 4.3 TARGETED PROTEIN DEGRADERS MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- 4.4 TARGETED PROTEIN DEGRADERS MARKET: EMERGING VS. DEVELOPED MARKETS
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Growing approvals and favorable regulatory environment
- 5.2.1.2 Multi-indication expansion
- 5.2.1.3 Capital inflows and big pharma tie-ups
- 5.2.1.4 Technological advancements in design and discovery
- 5.2.2 RESTRAINTS
- 5.2.2.1 Cost and scalability issues
- 5.2.2.2 Safety-related concerns
- 5.2.2.3 Intellectual property disputes
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Advancements in medicinal chemistry
- 5.2.3.2 Development of next-gen ligases with tissue-selective expressions
- 5.2.3.3 Increasing NDA filings for innovative degraders
- 5.2.4 CHALLENGES
- 5.2.4.1 Limited ligase expression in tissues
- 5.2.4.2 Strict regulatory guidelines
- 5.2.1 DRIVERS
- 5.3 TECHNOLOGY ANALYSIS
- 5.3.1 KEY TECHNOLOGIES
- 5.3.1.1 PROTACS (hetero-bifunctional)
- 5.3.1.2 Molecular glues/CELMODS
- 5.3.2 COMPLEMENTARY TECHNOLOGIES
- 5.3.2.1 Next-gen E3-ligase binder discovery
- 5.3.2.2 Nanoparticle/LNP delivery of degraders
- 5.3.3 ADJACENT TECHNOLOGIES
- 5.3.3.1 Antibody-drug conjugates (ADCs)
- 5.3.3.2 GENE editing/siRNA
- 5.3.1 KEY TECHNOLOGIES
- 5.4 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESSES
- 5.5 PRICING ANALYSIS
- 5.5.1 QUALITATIVE PRICING INSIGHTS FOR ELACESTRANT (ORSERDU), 2024
- 5.5.2 QUALITATIVE PRICING INSIGHTS FOR UPCOMING NOVEL TARGETED PROTEIN DEGRADERS
- 5.6 REIMBURSEMENT SCENARIO FOR ELACESTRANT (ORSERDU)
- 5.6.1 US
- 5.6.2 EUROPE
- 5.7 VALUE CHAIN ANALYSIS
- 5.8 ECOSYSTEM ANALYSIS
- 5.9 PATENT ANALYSIS
- 5.10 PIPELINE ANALYSIS
- 5.11 REGULATORY ANALYSIS
- 5.11.1 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS, BY REGION
- 5.11.2 REGULATORY FRAMEWORK
- 5.12 KEY CONFERENCES AND EVENTS, 2025-2026
- 5.13 PORTER'S FIVE FORCES ANALYSIS
- 5.13.1 THREAT OF NEW ENTRANTS
- 5.13.2 THREAT OF SUBSTITUTES
- 5.13.3 BARGAINING POWER OF SUPPLIERS
- 5.13.4 BARGAINING POWER OF BUYERS
- 5.13.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.14 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.14.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.14.2 KEY BUYING CRITERIA
- 5.15 INVESTMENT AND FUNDING SCENARIO
- 5.16 IMPACT OF AI/GEN AI ON TARGETED PROTEIN DEGRADERS MARKET
- 5.17 IMPACT OF 2025 US TARIFFS ON TARGETED PROTEIN DEGRADERS MARKET
- 5.17.1 INTRODUCTION
- 5.17.2 KEY TARIFF RATES
- 5.17.3 PRICE IMPACT ANALYSIS
- 5.17.4 IMPACT ON COUNTRY/REGION
- 5.17.4.1 North America
- 5.17.4.1.1 US
- 5.17.4.2 Europe
- 5.17.4.3 Asia Pacific
- 5.17.4.1 North America
- 5.17.5 IMPACT ON END-USE INDUSTRIES
6 TARGETED PROTEIN DEGRADERS MARKET, BY DEGRADER TYPE
- 6.1 INTRODUCTION
- 6.2 MOLECULAR GLUE
- 6.2.1 MEZIGDOMIDE (CC-92480)
- 6.2.1.1 Stronger cereblon binding and deeper substrate degradation to spur growth
- 6.2.2 IBERDOMIDE (CC-220)
- 6.2.2.1 Rising incidence of autoimmune diseases and cancer to boost market
- 6.2.1 MEZIGDOMIDE (CC-92480)
- 6.3 SERDS
- 6.3.1 ELACESTRANT
- 6.3.1.1 Increasing approvals to contribute to growth
- 6.3.2 GIREDESTRANT (GDC9545)
- 6.3.2.1 Growing prevalence of breast cancer to drive market
- 6.3.3 CAMIZESTRANT (AZD9833)
- 6.3.3.1 High potency and strong receptor degradation to bolster growth
- 6.3.1 ELACESTRANT
- 6.4 PROTAC
- 6.4.1 VEPDEGESTRANT (ARV-471)
- 6.4.1.1 Reliable systemic exposure and enhanced tumor targeting to support growth
- 6.4.2 LUXDEGALUTAMIDE
- 6.4.2.1 Favorable safety profile and early signals of efficacy to stimulate growth
- 6.4.3 BGB-16673
- 6.4.3.1 Higher efficacy and preference for oral formulation to aid growth
- 6.4.4 NX-5948
- 6.4.4.1 Advancements in next-gen oral BTK degraders targeting refractory B-cell malignancies to drive market
- 6.4.5 KT-474
- 6.4.5.1 Rising importance in treating autoimmune and inflammatory diseases to aid growth
- 6.4.1 VEPDEGESTRANT (ARV-471)
- 6.5 LDD/BIDAC
- 6.5.1 INCREASING USE OF LDD IN TUMOR-SPECIFIC DEGRADATION, HEMATOLOGIC CANCERS, AND IMMUNE MODULATION TO FUEL MARKET
- 6.6 LYTAC/ATAC
- 6.6.1 GROWING FOCUS ON CANCER IMMUNOTHERAPY TO DRIVE MARKET
- 6.7 AUTAC/ATTEC
- 6.7.1 GROWING USE OF AUTAC IN TREATING METABOLIC DISEASES AND RARE LYSOSOMAL STORAGE CONDITIONS TO BOOST MARKET
7 TARGETED PROTEIN DEGRADERS MARKET, BY THERAPEUTIC INDICATION
- 7.1 INTRODUCTION
- 7.2 ONCOLOGY
- 7.2.1 HIGH PREVALENCE OF MALIGNANCIES AND UNMET NEED FOR DURABLE THERAPIES TO PROMOTE GROWTH
- 7.3 INFLAMMATORY DISEASES
- 7.3.1 CHANGES IN LIFESTYLES AND ENVIRONMENTAL CONDITIONS TO AUGMENT GROWTH
- 7.4 OTHER DISEASES
8 TARGETED PROTEIN DEGRADERS MARKET, BY FORMULATION
- 8.1 INTRODUCTION
- 8.2 ORAL FORMULATIONS
- 8.2.1 TABLETS
- 8.2.1.1 Manufacturing scalability, stability, and patient convenience to aid growth
- 8.2.2 CAPSULES
- 8.2.2.1 Higher flexibility, faster development timelines, and enhanced bioavailability to foster growth
- 8.2.1 TABLETS
- 8.3 INJECTIONS
- 8.3.1 ESSENTIAL ROLE IN RAPID AND TARGETED DELIVERY OF SYSTEMIC TARGETED PROTEIN DEGRADERS TO BOOST MARKET
9 TARGETED PROTEIN DEGRADERS MARKET, BY END USER
- 9.1 INTRODUCTION
- 9.2 HOSPITALS & SPECIALTY CLINICS
- 9.2.1 ROBUST INFRASTRUCTURE AND MULTI-DISCIPLINARY CARE TEAMS TO EXPEDITE GROWTH
- 9.3 LONG-TERM CARE FACILITIES
- 9.3.1 INCREASING FOCUS ON QUALITY OF LIFE TO CONTRIBUTE TO GROWTH
- 9.4 HOME CARE SETTINGS
- 9.4.1 GROWING FOCUS ON CHRONIC DISEASE MANAGEMENT TO DRIVE MARKET
10 TARGETED PROTEIN DEGRADERS MARKET, BY REGION
- 10.1 INTRODUCTION
- 10.2 NORTH AMERICA
- 10.2.1 MACROECONOMIC ANALYSIS FOR NORTH AMERICA
- 10.2.2 US
- 10.2.2.1 Strong clinical pipeline and regulatory support to contribute to growth
- 10.2.3 CANADA
- 10.2.3.1 Favorable government initiatives to support growth
- 10.3 EUROPE
- 10.3.1 MACROECONOMIC ANALYSIS FOR EUROPE
- 10.3.2 GERMANY
- 10.3.2.1 Strong industry-academia partnership to foster growth
- 10.3.3 UK
- 10.3.3.1 Presence of leading academic institutions and translational research hubs to aid growth
- 10.3.4 FRANCE
- 10.3.4.1 Presence of leading biotechnology and diagnostic companies to spur growth
- 10.3.5 ITALY
- 10.3.5.1 Increasing innovations in PROTAC technology to promote growth
- 10.3.6 SPAIN
- 10.3.6.1 Robust academic network to contribute to growth
- 10.3.7 REST OF EUROPE
- 10.4 ASIA PACIFIC
- 10.4.1 MACROECONOMIC ANALYSIS FOR ASIA PACIFIC
- 10.4.2 CHINA
- 10.4.2.1 Surge in clinical trial activities to spur growth
- 10.4.3 JAPAN
- 10.4.3.1 Large geriatric population to contribute to growth
- 10.4.4 INDIA
- 10.4.4.1 Expanding contract development and manufacturing organization infrastructure to fuel market
- 10.4.5 SOUTH KOREA
- 10.4.5.1 Rapidly expanding pharmaceutical manufacturing infrastructure to propel market
- 10.4.6 AUSTRALIA
- 10.4.6.1 Streamlined clinical trial environment to accelerate growth
- 10.4.7 REST OF ASIA PACIFIC
- 10.5 LATIN AMERICA
- 10.5.1 MACROECONOMIC ANALYSIS FOR LATIN AMERICA
- 10.5.2 BRAZIL
- 10.5.2.1 Regulatory evolution supporting trial acceleration to drive market
- 10.5.3 MEXICO
- 10.5.3.1 Rising demand for novel therapeutics for chronic disease treatment to support growth
- 10.5.4 REST OF LATIN AMERICA
- 10.6 MIDDLE EAST & AFRICA
- 10.6.1 MACROECONOMIC ANALYSIS FOR MIDDLE EAST & AFRICA
- 10.6.2 GCC COUNTRIES
- 10.6.2.1 Saudi Arabia
- 10.6.2.1.1 Growing focus on life sciences sector to boost market
- 10.6.2.2 UAE
- 10.6.2.2.1 Emerging biotechnology sector to intensify growth
- 10.6.2.3 REST OF GCC countries
- 10.6.2.1 Saudi Arabia
- 10.6.3 REST OF MIDDLE EAST & AFRICA
11 COMPETITIVE LANDSCAPE
- 11.1 INTRODUCTION
- 11.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 11.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN TARGETED PROTEIN DEGRADERS MARKET
- 11.3 REVENUE ANALYSIS, 2028-2030
- 11.4 MARKET SHARE ANALYSIS, 2030
- 11.5 COMPANY VALUATION AND FINANCIAL METRICS
- 11.5.1 COMPANY VALUATION
- 11.5.2 FINANCIAL METRICS
- 11.6 BRAND/PRODUCT COMPARISON
- 11.7 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 11.7.1 STARS
- 11.7.2 EMERGING LEADERS
- 11.7.3 PERVASIVE PLAYERS
- 11.7.4 PARTICIPANTS
- 11.7.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 11.7.5.1 Company footprint
- 11.7.5.2 Region footprint
- 11.7.5.3 Degrader type footprint
- 11.7.5.4 Therapeutic indication footprint
- 11.7.5.5 Formulation footprint
- 11.8 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 11.8.1 PROGRESSIVE COMPANIES
- 11.8.2 RESPONSIVE COMPANIES
- 11.8.3 DYNAMIC COMPANIES
- 11.8.4 STARTING BLOCKS
- 11.8.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 11.8.5.1 Detailed list of key startups/SMEs
- 11.8.5.2 Competitive benchmarking of key startups/SMEs
- 11.9 COMPETITIVE SCENARIO
- 11.9.1 PRODUCT LAUNCHES AND APPROVALS
- 11.9.2 DEALS
- 11.9.3 EXPANSIONS
12 COMPANY PROFILES
- 12.1 KEY PLAYERS
- 12.1.1 BRYSTOL-MYERS SQUIBB COMPANY
- 12.1.1.1 Business overview
- 12.1.1.2 Products offered
- 12.1.1.3 Recent developments
- 12.1.1.3.1 Deals
- 12.1.1.3.2 Expansions
- 12.1.1.4 MnM view
- 12.1.1.4.1 Key strengths
- 12.1.1.4.2 Strategic choices
- 12.1.1.4.3 Weaknesses and competitive threats
- 12.1.2 THE MENARINI GROUP
- 12.1.2.1 Business overview
- 12.1.2.2 Products offered
- 12.1.2.3 Recent developments
- 12.1.2.3.1 Product launches and approvals
- 12.1.2.4 MnM view
- 12.1.2.4.1 Key strengths
- 12.1.2.4.2 Strategic choices
- 12.1.2.4.3 Weaknesses and competitive threats
- 12.1.3 ARVINAS
- 12.1.3.1 Business overview
- 12.1.3.2 Products offered
- 12.1.3.3 Recent developments
- 12.1.3.3.1 Deals
- 12.1.3.4 MnM view
- 12.1.3.4.1 Key strengths
- 12.1.3.4.2 Strategic choices
- 12.1.3.4.3 Weaknesses and competitive threats
- 12.1.4 BEONE MEDICINES
- 12.1.4.1 Business overview
- 12.1.4.2 Products offered
- 12.1.4.3 Recent developments
- 12.1.4.3.1 Deals
- 12.1.5 NURIX THERAPEUTICS, INC.
- 12.1.5.1 Business overview
- 12.1.5.2 Products offered
- 12.1.5.3 Recent developments
- 12.1.5.3.1 Deals
- 12.1.6 KYMERA THERAPEUTICS, INC.
- 12.1.6.1 Business overview
- 12.1.6.2 Products offered
- 12.1.6.3 Recent developments
- 12.1.6.3.1 Deals
- 12.1.7 C4 THERAPEUTICS, INC.
- 12.1.7.1 Business overview
- 12.1.7.2 Products offered
- 12.1.7.3 Recent developments
- 12.1.7.3.1 Deals
- 12.1.8 ASTRAZENECA
- 12.1.8.1 Business overview
- 12.1.8.2 Products offered
- 12.1.8.3 Recent developments
- 12.1.8.3.1 Deals
- 12.1.8.3.2 Expansions
- 12.1.9 F. HOFFMANN-LA ROCHE LTD
- 12.1.9.1 Business overview
- 12.1.9.2 Products offered
- 12.1.9.3 Recent developments
- 12.1.9.3.1 Deals
- 12.1.10 BAYER AG
- 12.1.10.1 Business overview
- 12.1.10.2 Products offered
- 12.1.10.3 Recent developments
- 12.1.10.3.1 Deals
- 12.1.11 CAPTOR THERAPEUTICS
- 12.1.11.1 Business overview
- 12.1.11.2 Products offered
- 12.1.11.3 Recent developments
- 12.1.11.3.1 Deals
- 12.1.12 RANOK THERAPEUTICS CO. LTD.
- 12.1.12.1 Business overview
- 12.1.12.2 Products offered
- 12.1.12.3 Recent developments
- 12.1.12.3.1 Product launches and approvals
- 12.1.13 PFIZER INC.
- 12.1.13.1 Business overview
- 12.1.13.2 Products offered
- 12.1.13.3 Recent developments
- 12.1.13.3.1 Product launches and approvals
- 12.1.14 NOVARTIS AG
- 12.1.14.1 Business overview
- 12.1.14.2 Products offered
- 12.1.14.3 Recent developments
- 12.1.14.3.1 Deals
- 12.1.15 FOGHORN THERAPEUTICS
- 12.1.15.1 Business overview
- 12.1.15.2 Products offered
- 12.1.15.3 Recent developments
- 12.1.15.3.1 Deals
- 12.1.1 BRYSTOL-MYERS SQUIBB COMPANY
- 12.2 OTHER PLAYERS
- 12.2.1 MONTE ROSA THERAPEUTICS
- 12.2.2 BIOTHERYX, INC.
- 12.2.3 CULLGEN
- 12.2.4 NEOMORPH
- 12.2.5 LYCIA THERAPEUTICS
- 12.2.6 PHOTYS THERAPEUTICS
- 12.2.7 PLEXIUM, INC.
- 12.2.8 SEED THERAPEUTICS, INC.
- 12.2.9 AVILAR THERAPEUTICS, INC.
- 12.2.10 AUTOMERA
13 APPENDIX
- 13.1 DISCUSSION GUIDE
- 13.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 13.3 CUSTOMIZATION OPTIONS
- 13.4 RELATED REPORTS
- 13.5 AUTHOR DETAILS



















