
|
시장보고서
상품코드
1737055
표적 단백질 분해 시장 : 분해 장치 유형별, 적응증별, 주요 지역별Targeted Protein Degradation Market Distribution by Type of Degrader, Target Indication, Key Geographical Regions (North America, Europe, Asia-Pacific, and Rest of the World ) |
||||||
세계의 표적 단백질 분해 시장 규모는 2035년까지 예측 기간 동안 32%의 연평균 복합 성장률(CAGR)로, 현재 4억 8,000만 달러에서 2035년까지 69억 4,000만 달러로 성장할 것으로 예측됩니다.
이 시장 세분화는 시장 규모와 기회 분석을 다음 매개 변수로 구분합니다.
분해장치 유형별
- SERD
- 프로택
- 분자 접착제
적응증별
- 유방암
- 다발성 골수종
주요 지역별
- 북미(미국, 캐나다)
- 유럽(영국, 독일, 프랑스, 이탈리아, 스페인)
- 아시아태평양(중국, 한국, 일본, 인도, 호주)
- 기타 지역(브라질, 이스라엘)
표적 단백질 분해 시장 : 성장과 동향
표적 단백질 분해(TPD)는 치료 곤란한 질환의 치료를 가능하게 할 가능성을 지닌 새로운 치료 수단입니다. 저분자 억제제나 단일클론항체 등의 종래의 의약품이 프로테옴의 20% 미만 밖에 표적으로 하지 않는 반면 TPD는 프로테옴 내의 미탐색 단백질의 대부분에 접근하는 새로운 접근법을 제공합니다. TPD를 통해 질병의 기능을 조절하거나 억제하기보다는, 그 양을 조절함으로써 질병에 대처할 수 있습니다. 단백질량의 제어는 단백질분해제라고 불리는 저분자 약물에 의해 달성됩니다.
저분자를 통한 선택적 TPD에는 기존의 신약 전략에는 없는 이점이 몇 가지 있습니다. 단백질 기능을 억제하여 표적 단백질 발현 증가를 억제할 수 있습니다. 단일 단백질 분해제가, 표적 단백질의 프로테아좀 분해를 통해, 복수의 병의 원인이 되는 단백질을 제거할 수 있을 가능성은 주목할 가치가 있습니다.
표적 단백질 분해 시장 : 주요 인사이트력
이 보고서는 표적 단백질 분해 시장의 현재 상태를 파악하고 업계 내 잠재적 성장 기회를 확인합니다.
- 285개 이상의 표적 단백질분해 요법이 승인되었거나, 다른 개발 단계에서 연구되고 있습니다.
- 평가되고 있는 표적 단백질 분해제의 20% 이상이 분자 접착제이며, 그 중 70% 이상이 암 질환의 치료를 목적으로 하고 있습니다.
- 35% 이상의 기술은 PROTACs의 개발에 초점을 맞추고 있으며, 이 중 38%는 CADD 해석을 이용하여 타겟을 식별하는 능력을 가지고 있습니다.
- 현재, 약 85개의 혁신적인 표적 단백질분해 기술이 안정적이고 효과적인 분해제의 개발에 이용 가능합니다.
- 현재, 다양한 지역에 걸쳐 다양한 질병의 치료를 목적으로 하는 표적 단백질분해 요법을 조사하기 위해 약 250건의 임상시험(등록 환자수는 약 63,000명)이 진행중입니다.
- 2019년 이후 1억 6,500만 달러 이상의 보조금이 NIH에서 수여되었으며, 이러한 보조금의 -80%는 1-4년의 지원 기간에 수여되었습니다.
- 지난 5년간 다양한 영향력 있는 저널에 여러 논문이 게재되었으며, 이러한 논문은 아시아태평양에서 더 많은 관심을 받고 있습니다.
- 이 분야에서 생성된 지적재산을 보호하기 위해 산업계 및 비산업계의 진입기업은 표적 단백질분해제 및 관련 기술에 관한 특허를 985건 이상 출원하고 있습니다.
- 관심 증가는 최근 다양한 이해관계자들 사이에서 맺어진 광범위한 파트너십에도 반영되었으며, 거래의 25%는 신규 단백질분해제의 연구개발에 초점을 맞추었습니다.
- 일부 투자자들은 표적 단백질분해 영역에서의 기회를 깨닫고 지난 6년간 다양한 자금 조달 라운드에서 약 135억 달러를 투자했습니다.
- 표적 단백질분해 시장은 치료 불가능한 표적을 선택적으로 분해하는 단백질분해제의 능력으로 향후 10년간 연평균 32%로 성장이 예상됩니다.
- 시장 추정 및 예측은 특히 미국, 프랑스, 스페인, 영국, 중국 등 다양한 국가에 분산될 것으로 예측됩니다.
표적 단백질 분해 시장 : 주요 부문
분해 장치 유형별로, 시장은 SERD, PROTAC, 분자 접착제로 분류됩니다. 현재 전이성 유방암에 대한 경구 SERD인 엘라세스트란트(ORSERDU)의 승인에 의해 표적 단백질분해 시장에서는 SERD 분야가 최대 점유율을 차지하고 있습니다. 또한 PROTAC 부문은 예측 기간 동안 더 빠른 속도로 성장할 것으로 보입니다.
표적 적응증별로 시장은 유방암과 다발성 골수종으로 구분됩니다. 현재 표적 단백질분해 시장에서 가장 높은 비율을 차지하고 있는 것은 유방암입니다. 이 동향은 새롭게 진단되는 사례의 12.5% 이상을 차지하는 유방암의 유병률 증가로 앞으로도 변하지 않을 것으로 보입니다. 게다가, 유방암 분절의 표적 단백질분해 시장은 예측 기간 동안 비교적 높은 CAGR로 성장할 가능성이 높습니다.
주요 지역별로 볼 때 시장은 북미, 유럽, 아시아태평양 및 기타 지역으로 구분됩니다. 현재 북미는 표적 단백질 분해 시장을 독점하고 있으며 최대 수익 점유율을 차지하고 있습니다. 게다가 아시아태평양 시장은 앞으로 수년간 더 높은 CAGR로 성장할 것으로 예측됩니다.
이 보고서는 세계의 표적 단백질 분해 시장을 조사했으며, 시장 개요와 함께 분해 장치 유형별, 적응증별, 주요 지역별 동향, 시장 진출기업 프로파일 등의 정보를 제공합니다.
목차
제1장 서문
제1장 1. 시장 개요
제2장 조사 방법
제3장 경제적 및 기타 프로젝트 특유의 고려 사항
제4장 주요 요약
제5장 소개
- 장의 개요
- 표적 단백질 분해의 개요
- 표적 단백질 분해 경로
- 단백질 분해제의 유형
- 대상 치료 영역
- 표적 단백질 분해에 수반하는 이점과 과제
- 장래의 전망
제6장 시장 상황 : 표적 단백질 분해 요법
- 장의 개요
- 표적단백분해요법: 시장정세
- 표적단백분해요법 개발자: 시장정세
제7장 시장 상황: 표적 단백질 분해 기술
- 장의 개요
- 표적단백분해기술: 시장정세
제8장 기업 프로파일
- 장의 개요
- 주요 진출기업의 상세 프로파일
- Arvinas
- Bristol-Myers Squibb
- C4 Therapeutics
- Loxo Oncology
- Olema Oncology
- Radius Health
- AstraZeneca
- Roche
- 기타 기업 프로파일
- BeiGene
- InnoCare Pharma
- Kangpu Biopharmaceuticals
- Kintor Pharmaceuticals
- Medivir
- Monte Rosa Therapeutics
- Ranok Therapeutics
- Sanofi
- Zentalis Pharmaceuticals
- Eisai Therapeutics
제9장 임상시험 분석
- 장의 개요
- 범위와 조사 방법
- 표적 단백질분해 : 임상시험 분석
제10장 조성금 분석
- 장의 개요
- 범위와 조사 방법
- 표적 단백질 분해 : 보조금 분석
제11장 특허 분석
- 장의 개요
- 범위와 조사 방법
- 표적 단백질 분해 : 특허 분석
- 특허 벤치마킹 분석
- 특허평가
- 인용수 상위의 특허
제12장 출판물 분석
제13장 파트너십 및 협업
제14장 자금조달과 투자
제15장 시장 영향 분석 : 촉진요인, 억제요인, 기회, 과제
제16장 세계의 표적 단백질 분해 시장
- 장의 개요
- 주요 전제와 조사 방법
- 세계의 표적 단백질 분해 시장, 예측(-2035년)
- 주요 시장 세분화
제17장 표적 단백질 분해 시장(분해 장치 유형별)
제18장 표적 단백질 분해 시장(표적 적응증별)
제19장 표적 단백질 분해 시장(주요 지역별)
제20장 표적 단백질 분해 시장, 의약품의 매출 예측
- 장의 개요
- 주요 전제와 조사 방법
- 상업화된 표적 단백질분해 시장 : 매출 예측
- 단계 III 표적 단백질 분해 시장 : 판매 예측
- 데이터의 삼각측량과 검증
제21장 세계의 표적 단백질 분해 기술 시장
제22장 주요 인사이트
제23장 결론
제24장 부록 1:표 형식 데이터
제25장 부록 2 : 기업 및 단체 일람
제26장 부록 3: 자금 조달 및 투자 목록
SHW 25.06.09TARGETED PROTEIN DEGRADATION MARKET: OVERVIEW
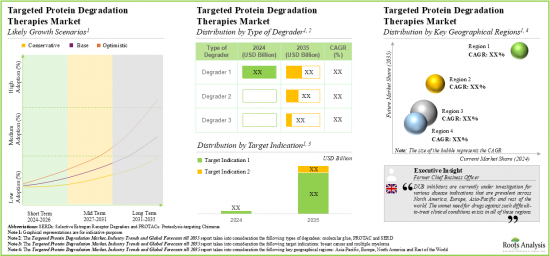
As per Roots Analysis, the global targeted protein degradation market is estimated to grow from USD 0.48 billion in the current year to USD 6.94 billion by 2035, at a CAGR of 32% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Degrader
- SERD
- PROTAC
- Molecular Glue
Target Indication
- Breast Cancer
- Multiple Myeloma
Key Geographical Regions
- North America (US and Canada)
- Europe (UK, Germany, France, Italy and Spain)
- Asia-Pacific (China, South Korea, Japan, India and Australia)
- Rest of the World (Brazil and Israel)
TARGETED PROTEIN DEGRADATION MARKET: GROWTH AND TRENDS
Targeted protein degradation (TPD) is an emerging therapeutic modality that has the potential to enable the treatment of difficult-to-treat diseases. While conventional medicines, such as small molecule inhibitors and monoclonal antibodies, target less than 20% of the proteome, TPD provides a novel approach to access the vast majority of unexplored proteins within the proteome. Through TPD, a disease can be addressed by controlling the amount of harmful protein rather than trying to modulate or inhibit its function. The control of protein levels is accomplished with a small molecule drug, called a protein degrader. TPD is a bifunctional small molecule that recruits E3 ubiquitin ligases to the protein of interest and mediates its ubiquitination and subsequent proteolysis by the proteasome.
Small molecule-mediated selective TPD has several advantages over traditional drug discovery strategies. One of the primary advantages is the high degree of target specificity that can be rapidly validated by leveraging the multiple layers of selectivity in the cellular machinery. Moreover, owing to the fact that the TPD degrader molecules cannot be destroyed, they can cause the destruction of many copies of harmful proteins and reduce systemic drug exposure. In addition, it can counteract increased target protein expression by inhibiting the protein function. It is worth noting that a single protein degrader has the ability to potentially eliminate multiple disease-causing proteins through proteasome degradation of the target protein. Owing to the above-mentioned advantages and the ongoing pace of technological advancements, the targeted protein degradation market is poised to experience noteworthy growth during the forecast period.
TARGETED PROTEIN DEGRADATION MARKET: KEY INSIGHTS
The report delves into the current state of the targeted protein degradation market and identifies potential growth opportunities within industry. Some key findings from the report include:
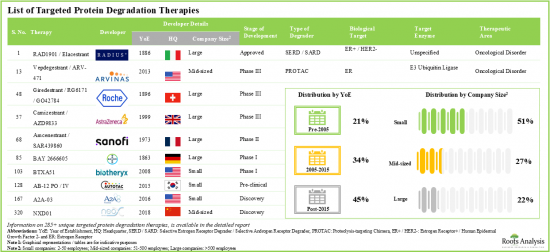
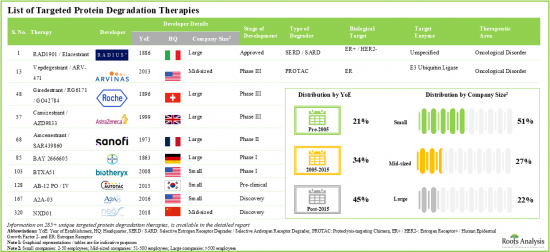
- 285+ targeted protein degradation therapies are either approved or being investigated in different stages of development; the market landscape features the presence of both established players and new entrants.
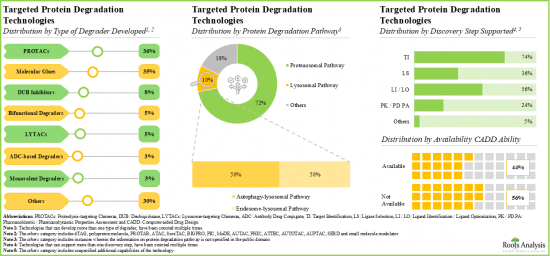
- Over 20% of the targeted protein degraders being evaluated are molecular glues; of these, more than 70% are intended for the treatment of oncological disorders.
- Over 35% of the technologies are focused on developing PROTACs; of these, 38% have the capability to identify the targets utilizing CADD analysis.
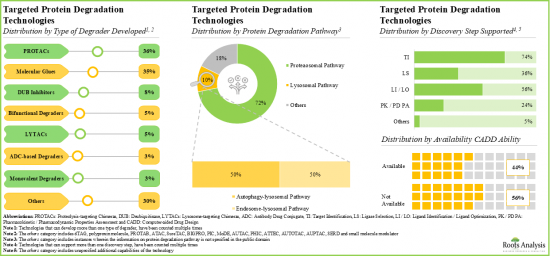
- At present, around 85 innovative targeted protein degradation technologies are available for developing stable and efficacious degraders; further, majority of the technology providers are start-ups / small businesses.
- Around 250 clinical trials (with around 63,000 enrolled patients) are currently underway to investigate targeted protein degradation therapies intended for treatment of various diseases, across different geographies.
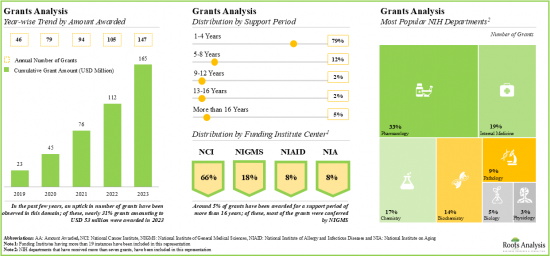
- Since 2019, grants worth over USD 165 million have been awarded by NIH; ~80% of these grants were awarded for a support period of 1-4 years.
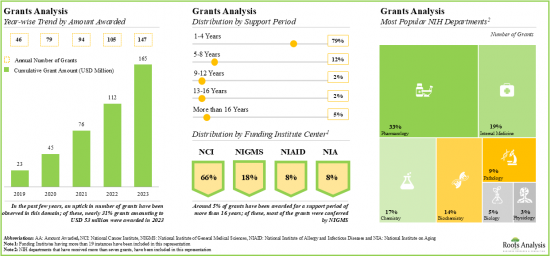
- In the last five years, several articles have been published in various high-impact journals; such publications have garnered more interest in the Asia-Pacific region.
- More than 985 patents have been filed for targeted protein degraders and related technologies by industry and non-industry players, to protect intellectual property generated within this field.
- The rising interest is reflected in the wide array of partnerships established between various stakeholders in the recent past; 25% of the deals were focused on research and development of novel protein degraders.
- Several investors have realized the opportunity within the domain of targeted protein degradation and invested around USD 13.5 billion across various funding rounds in the past six years.
- Owing to the protein degraders ability to degrade the undruggable targets selectively, the targeted protein degradation therapies market is anticipated to witness an annualized growth of 32% over the next decade.
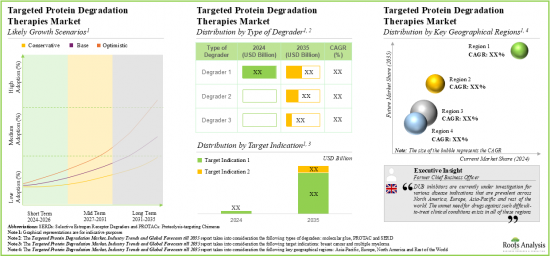
- The estimated market opportunity is projected to be distributed across different countries, especially in the US, France, Spain, the UK and China.
TARGETED PROTEIN DEGRADATION MARKET: KEY SEGMENTS
SERD Segment Occupies the Largest Share of the Targeted Protein Degradation Market
Based on the type of degrader, the market is segmented into SERD, PROTAC and molecular glue. At present, the SERD segment holds the maximum share of the targeted protein degradation market owing to the approval of elacestrant (ORSERDU), an oral SERD for metastatic breast cancer. Additionally, the PROTAC segment is likely to grow at a faster pace during the forecasted period.
By Target Indication, Breast Cancer is the Fastest Growing Segment of the Global Targeted Protein Degradation Market
Based on the target indication, the market is segmented into breast cancer and multiple myeloma. Currently, the breast cancer segment captures the highest proportion of the targeted protein degradation market. This trend is likely to remain the same in the future owing to the increasing prevalence of breast cancer, with over 12.5% of all newly diagnosed cases. Further, targeted protein degradation market for the breast cancer segment is likely to grow at a relatively higher CAGR during the forecast period.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific and Rest of the World. Currently, North America dominates the targeted protein degradation market and accounts for the largest revenue share. Further, the market in Asia-Pacific is expected to grow at a higher CAGR in the coming years.
Example Players in the Targeted Protein Degradation Market
- Arvinas
- AstraZeneca
- BeiGene
- Bristol-Myers Squibb
- C4 Therapeutics
- Eisai Therapeutics
- InnoCare Pharma
- Kangpu Biopharmaceuticals
- Kintor Pharmaceutical
- Loxo Oncology
- Medivir
- Monte Rosa Therapeutics
- Olema Oncology
- Radius Health
- Ranok Therapeutics
- Roche
- Sanofi
- Zentalis Pharmaceuticals
TARGETED PROTEIN DEGRADATION MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the targeted protein degradation market, focusing on key market segments, including [A] type of degrader, [B] target indication, and [C] key geographical regions.
- Targeted Protein Degradation Therapies Market Landscape: A comprehensive evaluation of the companies engaged in targeted protein degradation therapies market, based on several relevant parameters, such as [A] stage of development, [B] type of degrader, [C] biological target, [D] target enzyme, [E] target indication and [F] therapeutic area. Additionally, a comprehensive evaluation of the targeted protein degradation therapy developers, based on several relevant parameters, such as [G] year of establishment, [H] company size, [I] location of headquarters and [J] most active players.
- Targeted Protein Degradation Technologies Market Landscape: A comprehensive evaluation of the companies engaged in targeted protein degradation technologies market, based on several relevant parameters, such as [A] type of degrader developed, [B] protein degradation pathway, [C] site of degraded protein, [D] therapeutic area, [E] discovery step supported and [F] availability of computational modeling ability. Additionally, a comprehensive evaluation of the companies offering targeted protein degradation technologies, based on several relevant parameters, such as [G] year of establishment, [H] company size and [I] location of headquarters.
- Company Profiles: In-depth profiles of key companies engaged in the targeted protein degradation market, focusing on [A] overview of the company, [B] financial information (if available), [C] technology portfolio, [D] pipeline overview and [E] recent developments and an [F] informed future outlook.
- Clinical Trial Analysis: An insightful analysis of clinical trials related to targeted protein degradation, based on several parameters, such as [A] trial registration year, [B] number of patients enrolled, [C] trial phase, [D] trial status, [E] patient gender, [F] target indication, [G] study design, [H] most active sponsor / collaborator and [I] geography.
- Grants Analysis: A comprehensive assessment of grants that have been awarded to research institutes in the targeted protein degradation domain, based on various relevant parameters, such as [A] year of grant award, [B] amount awarded, [C] funding institute centers, [D] support period, [E] type of grant application, [F] purpose of grant award, [G] activity code, [H] study section involved, [I] popular NIH departments, [J] prominent program officers, [k] popular recipient organizations and [L] distribution of popular recipient organizations by states in the US.
- Patent Analysis: An in-depth analysis of patents filed / granted till date in the targeted protein degradation domain, based on various relevant parameters, such as [A] type of patent, [B] patent application year, [C] patent publication year, [D] patent jurisdiction, [E] CPC symbols, [F] type of applicant, [G] individual patent assignees, [H] patent age, [I] patent benchmarking, and [J] patent valuation analysis.
- Publication Analysis: An insightful analysis of peer-reviewed scientific articles related to research on targeted protein degradation, based on various relevant parameters, such as [A] year of publication, [B] type of publication, [C] key journal, [D] most active publisher, [E] most active copyright holder, [F] most popular keywords, [G] therapeutic area and [H] key geographical regions.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the targeted protein degradation market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] type of partner, [D] type of degrader, [E] most active players and [F] geographical distribution of the partnership activity.
- Funding and Investments: An in-depth analysis of the fundings raised by targeted protein degradation companies, based on relevant parameters, such as [A] year-wise trend of funding instances, [B] amount invested, [C] type of funding, [D] geographical analysis, [E] leading players and [F] leading investors.
- Market Impact Analysis: A thorough analysis of various factors, such as drivers, restraints, opportunities, and existing challenges that are likely to impact market growth.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
1.1. Market Overview
- 1.2. Market Share Insights
- 1.3. Key Market Insights
- 1.4. Report Coverage
- 1.5. Frequently Asked Questions
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.3. Project Methodology
- 2.4. Forecast Methodology
- 2.5. Robust Quality Control
- 2.6. Key Market Segmentations
- 2.7. Key Considerations
- 2.7.1. Demographics
- 2.7.2. Economic Factors
- 2.7.3. Government Regulations
- 2.7.4. Supply Chain
- 2.7.5. COVID Impact
- 2.7.6. Market Access
- 2.7.7. Healthcare Policies
- 2.7.8. Industry Consolidation
3. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Future Estimates
- 3.2.2. Currency Coverage and Foreign Exchange Rate
- 3.2.2.1. Major Currencies Affecting the Market
- 3.2.2.2. Factors affecting Currency Fluctuations and Foreign Exchange Rates
- 3.2.2.3. Impact of Foreign Exchange Rate Volatility on Market
- 3.2.2.4. Strategies For Mitigating Foreign Exchange Risk
- 3.2.3. Trade Policies
- 3.2.3.1. Impact of Trade Barriers on the Market
- 3.2.3.2. Strategies for Mitigating the Risks Associated with Trade Barriers
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. EXECUTIVE SUMMARY
5. INTRODUCTION
- 5.1. Chapter Overview
- 5.2. Overview of Targeted Protein Degradation
- 5.2.1. Historical Evolution of Targeted Protein Degraders
- 5.3. Targeted Protein Degrader Pathway
- 5.4. Types of Protein Degraders
- 5.4.1. Proteolysis Targeting Chimeras (PROTACs)
- 5.4.2. Endosome Targeting Chimeras (ENDTACs)
- 5.4.3. Photochemically Targeted Chimeras (PHOTACs)
- 5.4.4. Hydrophobic Tags
- 5.4.5. Immunomodulatory Imide Drugs (IMiDs)
- 5.4.6. Molecular Glues
- 5.4.7. Selective Hormone Receptor Degraders (SHRDs)
- 5.4.8. Specific and Nongenetic inhibitor-of-apoptosis proteins (IAP)- Dependent Protein Erasers (SNIPERs)
- 5.4.9. DUB Inhibitors
- 5.4.10. Other Protein Degraders
- 5.5. Targeted Therapeutic Areas
- 5.6. Benefits and Challenges Associated with Targeted Protein Degradation
- 5.7. Future Perspectives
6. MARKET LANDSCAPE: TARGETED PROTEIN DEGRADATION THERAPIES
- 6.1. Chapter Overview
- 6.2. Targeted Protein Degradation Therapies: Overall Market Landscape
- 6.2.1. Analysis by Stage of Development
- 6.2.2. Analysis by Type of Degrader
- 6.2.3. Analysis by Biological Target
- 6.2.4. Analysis by Target Enzyme
- 6.2.5. Analysis by Target Indication
- 6.2.6. Analysis by Therapeutic Area
- 6.3. Targeted Protein Degradation Therapy Developers: Overall Market Landscape
- 6.3.1. Analysis by Year of Establishment
- 6.3.2. Analysis by Company Size
- 6.3.3. Analysis by Location of Headquarters (Region)
- 6.3.4. Analysis by Year of Establishment and Location of Headquarters (Region)
- 6.3.5. Analysis by Company Size and Location of Headquarters (Region)
- 6.3.6. Most Active Developers: Distribution by Number of Therapies and Stage of Development
7. MARKET LANDSCAPE: TARGETED PROTEIN DEGRADATION TECHNOLOGIES
- 7.1. Chapter Overview
- 7.2. Targeted Protein Degradation Technologies: Overall Market Landscape
- 7.2.1. Analysis by Type of Degrader Developed
- 7.2.2. Analysis by Protein Degradation Pathway
- 7.2.3. Analysis by Site of Degraded Protein
- 7.2.4. Analysis by Therapeutic Area
- 7.2.5. Analysis by Discovery Step Supported
- 7.2.6. Analysis by Availability of Computational Modeling Ability
- 7.2.7. Analysis by Year of Establishment
- 7.2.8. Analysis by Company Size
- 7.2.9. Analysis by Location of Headquarters (Region)
- 7.2.10. Analysis by Year of Establishment and Location of Headquarters (Region)
- 7.2.11. Analysis by Company Size and Location of Headquarters (Region)
8. COMPANY PROFILES
- 8.1. Chapter Overview
- 8.2. Detailed Company Profiles of Prominent Players
- 8.2.1. Arvinas
- 8.2.1.1. Company Overview
- 8.2.1.2. Financial Information
- 8.2.1.3. Technology Portfolio
- 8.2.1.4. Pipeline Overview
- 8.2.1.5. Recent Developments and Future Outlook
- 8.2.2. Bristol-Myers Squibb
- 8.2.2.1. Company Overview
- 8.2.2.2. Financial Information
- 8.2.2.3. Technology Portfolio
- 8.2.2.4. Pipeline Overview
- 8.2.2.5. Recent Developments and Future Outlook
- 8.2.3. C4 Therapeutics
- 8.2.3.1. Company Overview
- 8.2.3.2. Financial Information
- 8.2.3.3. Technology Portfolio
- 8.2.3.4. Pipeline Overview
- 8.2.3.5. Recent Developments and Future Outlook
- 8.2.4. Loxo Oncology
- 8.2.4.1. Company Overview
- 8.2.4.2. Pipeline Overview
- 8.2.4.3. Recent Developments and Future Outlook
- 8.2.5. Olema Oncology
- 8.2.5.1. Company Overview
- 8.2.5.2. Pipeline Overview
- 8.2.5.3. Recent Developments and Future Outlook
- 8.2.6. Radius Health
- 8.2.6.1. Company Overview
- 8.2.6.2. Financial Information
- 8.2.6.3. Pipeline Overview
- 8.2.6.4. Recent Developments and Future Outlook
- 8.2.7. AstraZeneca
- 8.2.7.1. Company Overview
- 8.2.7.2. Financial Information
- 8.2.7.3. Pipeline Overview
- 8.2.7.4. Recent Developments and Future Outlook
- 8.2.8. Roche
- 8.2.8.1. Company Overview
- 8.2.8.2. Financial Information
- 8.2.8.3. Pipeline Overview
- 8.2.8.4. Recent Developments and Future Outlook
- 8.2.1. Arvinas
- 8.3. Short Profiles of Other Prominent Players
- 8.3.1. BeiGene
- 8.3.1.1. Company Overview
- 8.3.1.2. Pipeline Overview
- 8.3.2. InnoCare Pharma
- 8.3.2.1. Company Overview
- 8.3.2.2. Technology Portfolio
- 8.3.2.3. Pipeline Overview
- 8.3.3. Kangpu Biopharmaceuticals
- 8.3.3.1. Company Overview
- 8.3.3.2. Technology Portfolio
- 8.3.3.3. Pipeline Overview
- 8.3.4. Kintor Pharmaceuticals
- 8.3.4.1. Company Overview
- 8.3.4.2. Technology Portfolio
- 8.3.4.3. Pipeline Overview
- 8.3.5. Medivir
- 8.3.5.1. Company Overview
- 8.3.5.2. Technology Portfolio
- 8.3.5.3. Pipeline Overview
- 8.3.6. Monte Rosa Therapeutics
- 8.3.6.1. Company Overview
- 8.3.6.2. Technology Portfolio
- 8.3.6.3. Pipeline Overview
- 8.3.7. Ranok Therapeutics
- 8.3.7.1. Company Overview
- 8.3.7.2. Technology Portfolio
- 8.3.7.3. Pipeline Overview
- 8.3.8. Sanofi
- 8.3.8.1. Company Overview
- 8.3.8.2. Pipeline Overview
- 8.3.9. Zentalis Pharmaceuticals
- 8.3.9.1. Company Overview
- 8.3.9.2. Technology Portfolio
- 8.3.9.3. Pipeline Overview
- 8.3.10. Eisai Therapeutics
- 8.3.10.1. Company Overview
- 8.3.10.2. Pipeline Overview
- 8.3.1. BeiGene
9. CLINICAL TRIAL ANALYSIS
- 9.1. Chapter Overview
- 9.2. Scope and Methodology
- 9.3. Targeted Protein Degradation: Clinical Trial Analysis
- 9.3.1. Analysis by Trial Registration Year
- 9.3.2. Analysis of Number of Patients Enrolled by Trial Registration Year
- 9.3.3. Analysis by Trial Phase
- 9.3.4. Analysis of Number of Patients Enrolled by Trial Phase
- 9.3.5. Analysis by Trial Registration Year and Trial Phase
- 9.3.6. Analysis by Trial Status
- 9.3.7. Analysis by Patient Gender
- 9.3.8. Analysis by Target Indication
- 9.3.9. Analysis by Study Design
- 9.3.9.1. Analysis by Type of Trial Masking
- 9.3.9.2. Analysis by Type of Intervention Model
- 9.3.9.3. Analysis by Trial Purpose
- 9.3.9.4. Analysis by Design Allocation
- 9.3.10. Most Active Sponsor / Collaborator: Analysis by Number of Registered Trials
- 9.3.10.1. Analysis by Leading Industry Players
- 9.3.10.2. Analysis by Leading Non-Industry Players
- 9.3.11. Analysis by Geography
- 9.3.11.1. Analysis of Clinical Trials by Trial Status and Geography
- 9.3.11.2. Analysis of Patients Enrolled by Trial Status and Geography
10. GRANTS ANALYSIS
- 10.1. Chapter Overview
- 10.2. Scope and Methodology
- 10.3. Targeted Protein Degradation: Grants Analysis
- 10.3.1. Analysis by Year of Grant Award
- 10.3.2. Analysis by Amount Awarded
- 10.3.3. Analysis by Funding Institute Center
- 10.3.4. Analysis by Support Period
- 10.3.5. Analysis by Funding Institute Center and Support Period
- 10.3.6. Analysis by Type of Grant Application
- 10.3.7. Analysis by Purpose of Grant Award
- 10.3.8. Analysis by Activity Code
- 10.3.9. Analysis by Study Section Involved
- 10.3.10. Most Popular NIH Departments: Analysis by Number of Grants
- 10.3.11. Prominent Program Officers: Analysis by Number of Grants
- 10.3.12. Popular Recipient Organizations: Analysis by Number of Grants
- 10.3.13. Popular Recipient Organizations: Analysis by Grant Amount
- 10.3.14. Popular Recipient Organizations: Analysis by States in the US
11. PATENT ANALYSIS
- 11.1. Chapter Overview
- 11.2. Scope and Methodology
- 11.3. Targeted Protein Degradation: Patents Analysis
- 11.3.1. Analysis by Patent Publication Year
- 11.3.2. Analysis by Patent Application Year
- 11.3.3. Analysis of Granted Patents and Patent Applications by Publication Year
- 11.3.4. Analysis by Patent Jurisdiction
- 11.3.5. Analysis by CPC Symbols
- 11.3.6. Analysis by Type of Applicant
- 11.3.7. Leading Industry Players: Analysis by Number of Patents
- 11.3.8. Leading Non-Industry Players: Analysis by Number of Patents
- 11.3.9. Leading Patent Assignees: Analysis by Number of Patents
- 11.4. Patent Benchmarking Analysis
- 11.4.1. Analysis of Patent Characteristics (CPC Codes) by Leading Industry Players
- 11.5. Patent Valuation
- 11.6. Leading Patents by Number of Citations
12. PUBLICATION ANALYSIS
- 12.1. Chapter Overview
- 12.2. Scope and Methodology
- 12.3. Targeted Protein Degradation: Publication Analysis
- 12.3.1. Analysis by Year of Publication
- 12.3.2. Analysis by Type of Publication
- 12.3.3. Key Journals: Analysis by Number of Publications
- 12.3.4. Key Journals: Analysis by Journal Impact Factor
- 12.3.5. Most Active Publisher: Analysis by Number of Publications
- 12.3.6. Most Active Copyright Holder: Analysis by Number of Publications
- 12.3.7. Analysis by Emerging Focus Area
- 12.3.8. Analysis by Therapeutic Area
- 12.3.9. Analysis by Geography
13. PARTNERSHIPS AND COLLABORATIONS
- 13.1. Chapter Overview
- 13.2. Partnership Models
- 13.3. Targeted Protein Degradation: Partnerships and Collaborations
- 13.3.1. Analysis by Year of Partnership
- 13.3.2. Analysis by Type of Partnership
- 13.3.3. Analysis by Year and Type of Partnership
- 13.3.4. Analysis by Type of Partner
- 13.3.5. Analysis by Type of Degrader
- 13.3.6. Most Active Players: Analysis by Number of Partnerships
- 13.4. Analysis by Geography
- 13.4.1. Local and International Agreements
- 13.4.2. Intercontinental and Intracontinental Agreements
14. FUNDING AND INVESTMENTS
- 14.1. Chapter Overview
- 14.2. Funding Models
- 14.3. Targeted Protein Degradation: Funding and Investments
- 14.3.1. Analysis by Year of Funding
- 14.3.2. Analysis by Amount Invested
- 14.3.3. Analysis by Type of Funding
- 14.3.4. Analysis by Amount Invested and Type of Funding
- 14.3.5. Analysis of Amount Invested by Year and Type of Funding
- 14.3.6. Analysis by Geography
- 14.3.7. Most Active Players
- 14.3.7.1. Analysis by Number of Funding Instances
- 14.3.7.2. Analysis by Amount Invested
- 14.3.8. Leading Investors: Analysis by Number of Funding Instances
- 14.4. Concluding Remarks
15. MARKET IMPACT ANALYSIS: DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES
- 15.1. Chapter Overview
- 15.2. Market Drivers
- 15.3. Market Restraints
- 15.4. Market Opportunities
- 15.5. Market Challenges
- 15.6. Conclusion
16. GLOBAL TARGETED PROTEIN DEGRADATION THERAPIES MARKET
- 16.1. Chapter Overview
- 16.2. Key Assumptions and Methodology
- 16.3. Global Targeted Protein Degradation Therapies Market, Forecasted Estimates (Till 2035)
- 16.3.1. Scenario Analysis
- 16.3.1.1. Conservative Scenario
- 16.3.1.2. Optimistic Scenario
- 16.3.1. Scenario Analysis
- 16.4. Key Market Segmentations
17. TARGETED PROTEIN DEGRADATION THERAPIES MARKET, BY TYPE OF DEGRADER
- 17.1. Chapter Overview
- 17.2. Key Assumptions and Methodology
- 17.3. Targeted Protein Degradation Therapies Market: Distribution by Type of Degrader
- 17.3.1. Targeted Protein Degradation Therapies Market for SERDs: Forecasted Estimates (Till 2035)
- 17.3.2. Targeted Protein Degradation Therapies Market for PROTACs: Forecasted Estimates (Till 2035)
- 17.3.3. Targeted Protein Degradation Therapies Market for Molecular Glues: Forecasted Estimates (Till 2035)
- 17.4. Data Triangulation and Validation
18. TARGETED PROTEIN DEGRADATION THERAPIES MARKET, BY TARGET INDICATION
- 18.1. Chapter Overview
- 18.2. Key Assumptions and Methodology
- 18.3. Targeted Protein Degradation Therapies Market: Distribution by Target Indication
- 18.3.1. Targeted Protein Degradation Therapies Market for Breast Cancer: Forecasted Estimates (Till 2035)
- 18.3.2. Targeted Protein Degradation Therapies Market for Multiple Myeloma: Forecasted Estimates (Till 2035)
- 18.4. Data Triangulation and Validation
19. TARGETED PROTEIN DEGRADATION THERAPIES MARKET, BY KEY GEOGRAPHICAL REGIONS
- 19.1. Chapter Overview
- 19.2. Key Assumptions and Methodology
- 19.3. Targeted Protein Degradation Therapies Market: Distribution by Key Geographical Regions
- 19.3.1. Targeted Protein Degradation Therapies Market in North America: Forecasted Estimates (Till 2035)
- 19.3.1.1. Targeted Protein Degradation Therapies Market in US: Forecasted Estimates (Till 2035)
- 19.3.1.2. Targeted Protein Degradation Therapies Market in Canada: Forecasted Estimates (Till 2035)
- 19.3.2. Targeted Protein Degradation Therapies Market in Europe: Forecasted Estimates (Till 2035)
- 19.3.2.1. Targeted Protein Degradation Therapies Market in UK: Forecasted Estimates (Till 2035)
- 19.3.2.2. Targeted Protein Degradation Therapies Market in Germany: Forecasted Estimates (Till 2035)
- 19.3.2.3. Targeted Protein Degradation Therapies Market in France: Forecasted Estimates (Till 2035)
- 19.3.2.4. Targeted Protein Degradation Therapies Market in Italy: Forecasted Estimates (Till 2035)
- 19.3.2.5. Targeted Protein Degradation Therapies Market in Spain: Forecasted Estimates (Till 2035)
- 19.3.3. Targeted Protein Degradation Therapies Market in Asia-Pacific: Forecasted Estimates (Till 2035)
- 19.3.3.1. Targeted Protein Degradation Therapies Market in China: Forecasted Estimates (Till 2035)
- 19.3.3.2. Targeted Protein Degradation Therapies Market in South Korea: Forecasted Estimates (Till 2035)
- 19.3.3.3. Targeted Protein Degradation Therapies Market in India: Forecasted Estimates (Till 2035)
- 19.3.3.4. Targeted Protein Degradation Therapies Market in Japan: Forecasted Estimates (Till 2035)
- 19.3.3.5. Targeted Protein Degradation Therapies Market in Australia: Forecasted Estimates (Till 2035)
- 19.3.4. Targeted Protein Degradation Therapies Market in Rest of the World: Forecasted Estimates (Till 2035)
- 19.3.4.1. Targeted Protein Degradation Therapies Market in Brazil: Forecasted Estimates (Till 2035)
- 19.3.4.2. Targeted Protein Degradation Therapies Market in Israel: Forecasted Estimates (Till 2035)
- 19.3.1. Targeted Protein Degradation Therapies Market in North America: Forecasted Estimates (Till 2035)
- 19.5. Data Triangulation and Validation
20. TARGETED PROTEIN DEGRADATION THERAPIES MARKET, SALES FORECAST OF DRUGS
- 20.1. Chapter Overview
- 20.2. Key Assumptions and Methodology
- 20.3. Commercialized Targeted Protein Degradation Therapies Market: Sales Forecast
- 20.3.1. RAD1901 / Elacestrant / Orserdu Sales Forecast
- 20.4. Phase III Targeted Protein Degradation Therapies Market: Sales Forecast
- 20.4.1. Vepdegestrant / ARV-471 Sales Forecast
- 20.4.2. Camizestrant / AZD9833 Sales Forecast
- 20.4.3. CC-92480 / BMS-986348 / Mezigdomide Sales Forecast
- 20.4.4. LY3484356 / Imlunestrant Sales Forecast
- 20.4.5. OP-1250 / OP-1250-301 / Palazestrant Sales Forecast
- 20.5. Data Triangulation and Validation
21. GLOBAL TARGETED PROTEIN DEGRADATION TECHNOLOGIES MARKET
- 21.1. Chapter Overview
- 21.2. Key Assumptions
- 21.3. Forecast Methodology
- 21.4. Global Targeted Protein Degradation Technologies Market, Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 21.4.1. Scenario Analysis
- 21.4.1.1. Conservative Scenario
- 21.4.1.2. Optimistic Scenario
- 21.4.1. Scenario Analysis
- 21.5. Targeted Protein Degradation Technologies Market: Distribution by Type of Payment Model Employed
- 21.5.1. Targeted Protein Degradation Technologies Market for Upfront Payments: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 21.5.2. Targeted Protein Degradation Technologies Market for Milestone Payments: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 21.6. Data Triangulation and Validation
22. EXECUTIVE INSIGHTS
- 22.1. Chapter Overview
- 22.2. GlycoEra
- 22.2.1. Company Snapshot
- 22.2.2. Interview Transcript: Ganesh Kaundinya, Chief Executive Officer and President
- 22.3. Polyprox Therapeutics
- 22.3.1. Company Snapshot
- 22.3.2. Interview Transcript: Laura Itzhaki, Chief Scientific Officer and Founder
- 22.4. Xios Therapeutics
- 22.4.1. Company Snapshot
- 22.4.2. Interview Transcript: Louise Bergeron, Former Chief Scientific Officer and Former Vice President, Research
- 22.5. Mission Therapeutics
- 22.5.1. Company Snapshot
- 22.5.2. Interview Transcript: Paul Wallace, Former Chief Business Officer
- 22.6. Ubiquigent
- 22.6.1. Company Snapshot
- 22.6.2. Interview Transcript: Jason Brown, Former Scientific and Business Development Director
- 22.7. Almac Discovery
- 22.7.1. Company Snapshot
- 22.7.2. Interview Transcript: Martin Wales, Former Vice President, Business Development and Licensing and Gerald Gavory, Former Vice President, Biology
- 22.8. University of Delaware
- 22.8.1. Company Snapshot
- 22.8.2. Interview Transcript: Zhihao Zhuang (Professor)
- 22.9 Francis Crick Institute
- 22.9.1. Company Snapshot
- 22.9.2. Interview Transcript: Katrin Rittinger (Group Leader)
- 22.10 Anonymous
- 22.10.1. Interview Transcript: Chief Scientific Officer
- 22.11 Anonymous
- 22.11.1. Interview Transcript: Director of Oncology Research



















