
|
시장보고서
상품코드
1440357
전립선특이항원(PSA) 검사 : 세계 시장 점유율 분석, 업계 동향과 통계, 성장 예측(2024-2029년)Global Prostate Specific Antigen (PSA) Test - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
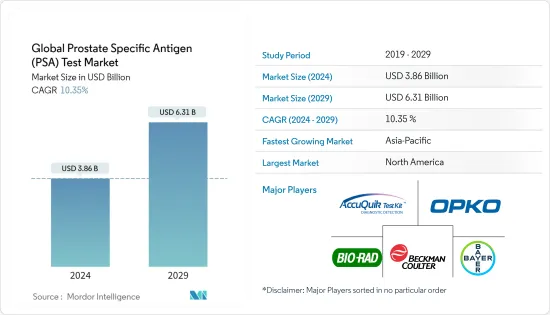
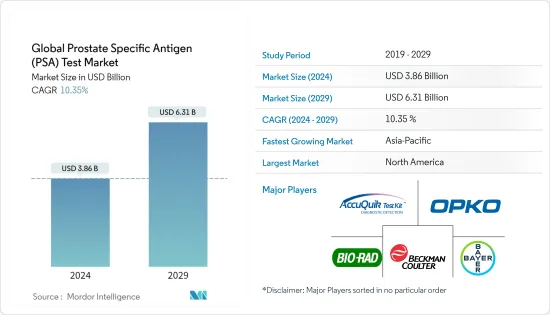
세계의 전립선특이항원(PSA) 검사 시장 규모는 2024년에 38억 6,000만 달러로 추정되며, 2029년까지 63억 1,000만 달러에 달할 것으로 예측되고 있으며, 예측 기간(2024-2029년) 중 10.35%의 CAGR로 성장합니다.
신종 코로나바이러스 감염증(COVID-19) 사태는 암과 같은 신종 코로나바이러스 이외의 질병에도 영향을 미치고 있습니다. 신종 코로나바이러스 감염증(COVID-19)의 첫 번째 파동으로 전립선암 진단 건수가 감소했습니다. 이 추세는 2020년 하반기에 거의 회복되었습니다. 그러나 그 수는 여전히 예상보다 낮았습니다. 2022년 4월에 발표된 '네덜란드에서 신종 코로나바이러스 감염증(COVID-19)이 전립선암 치료에 미치는 영향'이라는 제목의 연구에 따르면 신종 코로나바이러스 감염증(COVID-19)의 첫 번째 파동에서 전립선암 진단이 17% 감소했습니다. 5월 이후 진단 건수는 2020년 말까지 예측된 수치의 약 95%를 회복하기 시작했습니다. 이로 인해 공급망의 혼란이 관찰되었고, 시장 관계자들은 많은 문제에 직면했습니다. 그러나 암 치료와 높은 수준의 진단 및 치료 수준을 유지하는 것은 전 세계 국가 의료 기관 및 국제 의료 시스템의 주요 우선 순위가되었습니다.
전립선암 발병률 증가는 진단 제품 수요를 촉진하는 주요 요인입니다. 전립선암은 피부암을 제외하고 남성에게 가장 흔한 암입니다. 2022년 8월 미국임상종양학회(ASCO)의 최신 정보에 따르면 전립선암은 세계에서 4번째로 많이 진단되는 암입니다. ASCO에 따르면 2021년 미국에서 약 268,490명의 남성이 전립선암 진단을 받았다고 합니다. 같은 자료에 따르면 2020년에는 약 1,414,259명이 전립선암 진단을 받은 것으로 추정됩니다. 예측 기간 중 시장 성장을 지원할 것으로 예상되는 제품.
기술적으로 진보된 제품 개발도 시장을 주도하고 있습니다. 예를 들어 휴대가 간편한 신속한 전립선암 검진 키트는 의료 접근성이 제한된 아프리카계 미국인 남성 등 전립선암 발병률이 높은 지역사회에 조기 경고를 제공할 수 있습니다. 이 저비용 개념 증명 테스트는 테스트 스트립과 소형 큐브형 1.6인치 리더를 결합하여 혈액 한 방울에서 전립선 특이 항원(PSA)으로 알려진 전립선암 마커를 몇 분 안에 정량화할 수 있도록 합니다.
또한 전립선암 진단을 개선하기 위한 여러 연구 시작이 시장 확대를 돕고 있습니다. 예를 들어 영국 전역의 새로운 병원은 2021년9월에 인공지능을 사용하여 전립선암 샘플 분석을 개선하기위한 획기적인 시험에 참여할 예정입니다. 6개의 새로운 NHS 트러스트가 이 획기적인 시험을 시작할 것으로 예상되며, 이 획기적인 시험은 1억 4천만 유로 규모의 NHSX AI in Health and Care 상(NHSX AI in Health and Care award)의 일부로 자금이 지원될 예정입니다. Galen Prostate는 헬스케어 스타트업인 Ibex Medical Analytics가 개발한 AI 기술의 이름입니다. 이 자금을 통해 병원은 14개월에 걸쳐 600명의 남성에게서 채취한 생검을 사용하여 병리학자들의 연구와 AI 결과를 비교할 수 있게 됩니다. AI 기술은 진단 기간을 단축하고 생검 분석의 정확성을 향상시켜 임상의의 중요한 시간을 확보할 수 있도록 도와줍니다.
또한 시장 관계자들도 시장 성장에 기여하기 위한 노력을 기울이고 있습니다. 예를 들어 2021년 6월 케임브리지의 스타트업 루시다 메디컬(Lucida Medical)은 GE헬스케어가 Wayra UK와 함께 헬스케어용 AI 솔루션을 만드는 초기 단계의 기술적으로 정교한 기업을 촉진하기 위해 만든 프로그램인 Edison Accelerator에 합류했다고 발표했습니다. 루시다 메디컬의 에디슨 엑셀러레이터 프로그램 참여는 MRI 분석을 통해 전립선암을 보다 정확하게 검출하는 기술을 통해 암 진단 경로를 파괴하여 방사선과 의사의 시간을 절약하고 환자가 최상의 진단과 치료를 받을 수 있도록 하겠다는 루시다 메디컬의 목표를 향한 중요한 발걸음입니다.
시장에서의 제품 가용성도 시장 성장에 기여했습니다. 예를 들어 2020년 3월 스위스 암 진단 기업 프로테오메딕스(Proclarix)가 전립선암 진단을 위한 혈액 기반 검사 'Proclarix'를 유럽에서 시판했습니다.
따라서 위의 요인으로 인해 시장은 예측 기간 중 성장세를 보일 것으로 예상됩니다. 그러나 높은 진단 비용은 시장 성장을 크게 저해하고 있습니다.
전립선특이항원(PSA) 검사 시장 동향
예비검사 부문은 전립선특이항원(PSA) 검사 시장에서 주요 시장 점유율을 차지할 것으로 예상
PSA 검사가 더 널리 보급되면서 조기 발견과 치료가 가능해져 생존율이 높아졌습니다. PSA 검사는 이전에 발견되지 않은 작은 병변을 발견하여 더 진행된 암 단계로 진행될 수도 있고 그렇지 않을 수도 있습니다. 50세 이상의 대부분의 남성과 이 질병에 걸릴 위험이 있는 사람은 심각한 증상이 나타나기 전에 질병을 진단하는 데 도움이 되므로 이 검사를 받아야 합니다.
남성 전립선암 발생률이 증가함에 따라 고급 진단 제품의 필요성이 대두되고 있으며, 이는 시장 성장을 가속하고 있습니다. 전립선암은 미국 남성에게 가장 흔한 암입니다. 2022년 1월 미국암협회의 최신 정보에 따르면 2022년에는 미국에서 약 268,490명의 전립선암 환자가 새로 진단될 것으로 추정됩니다.
또한 Globocan 2020에 따르면 전립선암은 남성 인구에서 두 번째로 많이 발생하는 암입니다. 같은 자료에 따르면 전립선암의 유병률은 1,414,259명으로 전 세계 암 환자 수의 7.3%를 차지합니다. 전립선암은 노년층에서 많이 발생하기 때문에 전 세계 고령화 인구 증가도 시장 성장을 가속할 것으로 예상됩니다. 예를 들어 유엔이 발표한 2020년 세계 인구 고령화 하이라이트에 따르면 2020년 전 세계 노인 인구(65세 이상)는 7억 2,700만 명으로 2050년 말에는 15억 명에 달할 것으로 예상됩니다. 따라서 전립선암 환자 수 증가는 예비 검사에 대한 수요를 증가시켜이 부문의 성장을 가속할 것으로 예상됩니다.
앞서 언급한 요인으로 인해 예비 테스트 부문은 예측 기간 중 크게 발전할 것으로 예상됩니다.
북미는 시장에서 중요한 점유율을 차지할 것으로 예상되며, 예측 기간 중에도 마찬가지일 것으로 예상
북미는 전립선암의 유병률 증가와 더불어 전립선암에 대한 인식이 높아지고 연구개발이 증가함에 따라 시장 점유율이 크게 증가할 것으로 예상됩니다. 그러나 현재 신종 코로나바이러스 감염증(COVID-19)이 미국내 암 치료에 미치는 영향은 새로운 암 발견과 치료 제공의 감소와 지연을 초래하고 있습니다. 2021년 10월에 발표된 연구 논문 'COVID-19가 암 치료에 미치는 영향: COVID-19가 미국 노인의 암 진단과 치료를 지연시키는 방법'에 따르면 2020년에는 2019년 대비 전립선암 검진 건수가 크게 감소한 것으로 나타났습니다. 가장 큰 감소는 4월에 발생한 전립선 검진(56%)이었습니다. 따라서 이 지역의 전립선암 부담은 증가할 것으로 예상되며, 이는 예측 기간 중 시장 성장을 가속할 것으로 예상됩니다.
시장 확대는 미국 정부의 적극적인 지원으로 더욱 촉진되고 있습니다. 예를 들어 Datar Cancer Genetics는 2022년 2월 미국 식품의약국(FDA)이 조기 전립선암을 식별하기 위해 개발된 자사의 TriNetra-Prostate 혈액 검사를 획기적인 기기로 지정했다고 발표했습니다. Paige Prostate는 전립선 생검 사진에서 악성 위험 영역을 감지하기 위해 개발된 최초의 인공지능(AI) 기반 프로그램으로 2021년 9월 미국 식품의약국(FDA)의 승인을 받았습니다. 이 프로그램을 통해 병리학자들은 다음을 수행할 수 있습니다. 더 정확한 악성 조직 진단을 할 수 있으며, 아마도 전 세계에서 증가하는 암 사례에 대처하는 데 도움이 될 것입니다.
앞서 언급한 요인으로 인해 이 지역은 예측 기간 중 상당한 성장을 보일 것으로 예상됩니다.
전립선특이항원(PSA) 검사 산업 개요
전립선 특이 항원(PSA) 검사 시장은 세분화되어 있고 경쟁이 치열하며 여러 주요 기업으로 구성되어 있습니다. Accuquik Test Kits, Bayer AG, Bio-Rad Laboratories, Inc., Beckman Coulter, Inc. Company, Abcam PLC, Lomina AG, Laboratory Corporation of America Holdings, OPKO Health, Hanzhou Testsea biotechnology, and Proteomedix.
기타 혜택
- 엑셀 형식의 시장 예측(ME) 시트
- 3개월간의 애널리스트 지원
목차
제1장 서론
- 조사의 전제조건과 시장의 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 촉진요인
- 전립선암의 이환율 증가
- 증가하는 정부의 구상
- 기술의 진보
- 시장 억제요인
- 고액의 진단 비용
- Porter's Five Forces 분석
- 신규 진출업체의 위협
- 구매자의 교섭력
- 공급 기업의 교섭력
- 대체품의 위협
- 경쟁 기업간 경쟁의 강도
제5장 시장 세분화
- 검사 유형별
- 예비 테스트
- 확인 검사
- PCA3 테스트
- 경직장 초음파 검사
- 생검
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카공화국
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 개요
- Accuquik Test Kits
- Bayer AG
- Bio-Rad Laboratories, Inc.
- Beckman Coulter, Inc.
- Fujirebio(HU Group company)
- General Electric Company
- Abcam plc.
- Lomina AG.
- Laboratory Corporation of America Holdings
- OPKO Health, Inc.
- Hanzhou Testsea biotechnology co., LTD.
- Proteomedix
제7장 시장 기회와 향후 동향
KSA 24.03.12The Global Prostate Specific Antigen Test Market size is estimated at USD 3.86 billion in 2024, and is expected to reach USD 6.31 billion by 2029, growing at a CAGR of 10.35% during the forecast period (2024-2029).
The COVID-19 outbreak has impacted non-COVID illnesses, such as cancer. The number of prostate cancer diagnoses decreased during the first COVID-19 wave. This trend was mostly restored in the second half of 2020. However, the number remained lower than predicted. According to a study released in April 2022 titled "Impact of the COVID-19 Outbreak on Prostate Cancer Care in the Netherlands" the first COVID-19 wave saw a 17 percent decrease in prostate cancer diagnoses. From May onwards, the number of diagnoses began to return to around 95% of what was projected by the end of 2020. Hence, market players faced numerous challenges as disruptions in the supply chain were observed. However, cancer care and maintaining high standards of diagnosis and treatment have been the major priorities of the national healthcare bodies and international healthcare systems worldwide.
The increasing prevalence of prostate cancer is a major factor that propels the demand for diagnostic products. Prostate cancer is the most common cancer among men, except for skin cancer. As per the American Society of Clinical Oncology (ASCO) updates from August 2022, prostate cancer is the world's fourth most commonly diagnosed cancer. As per the ASCO, an estimated 268,490 men in the United States were diagnosed with prostate cancer in 2021. Also, according to the same source, an estimated 1,414,259 people were diagnosed with prostate cancer in 2020. Hence, this creates a need for an advanced product that is projected to support the market growth over the forecast period.
The development of technologically advanced products is also driving the market. For example, a highly portable and quick prostate cancer screening kit could provide early warning to communities with a higher incidence of prostate cancer, such as African American males with limited health care access. The low-cost proof-of-concept test combines a test strip and a compact cube-shaped 1.6-inch reader to quantify a prostate cancer marker known as the prostate-specific antigen (PSA) from a drop of blood in minutes.
In addition, the launch of several studies to better prostate cancer diagnostics is aiding market expansion. For instance, new hospitals throughout England planned to join a groundbreaking trial in September 2021 that attempts to improve the analysis of prostate cancer samples using Artificial Intelligence. Six new NHS trusts were thought to kick-start this groundbreaking trial, which will be funded as part of the Eur140 million NHSX AI in Health and Care awards. Galen Prostate is the name of the AI technology developed by Ibex Medical Analytics, a health tech startup. The money will allow hospitals to compare the AI results against a pathologist's study using biopsies from 600 males over 14 months. AI technology can shorten diagnosis timelines, increase biopsy analysis accuracy, and free up clinicians' important time.
Besides, market players are also taking initiatives that are contributing to the market's growth. For instance, in June 2021, Lucida Medical, a Cambridge startup, announced that it had joined the Edison Accelerator, a program created by GE Healthcare in collaboration with Wayra UK to promote early-stage and technologically sophisticated enterprises creating AI solutions for healthcare. Lucida Medical's participation in the Edison Accelerator program is a significant step toward the company's goal of disrupting the cancer diagnostic pathway with technology that more accurately detects prostate cancer by analyzing MRI, allowing radiologists to save time and patients to receive the best possible diagnosis and treatment.
The availability of products in the market has also contributed to the market growth. For instance, in March 2020, Proclarix, a blood-based test for prostate cancer diagnosis, was made commercially available in Europe by Proteomedix, a Swiss cancer diagnostics business.
Thus, owing to the abovementioned factors, the market is expected to show growth over the forecast period. However, the high cost of diagnosis significantly hinders the market's growth.
Prostate Specific Antigen Test Market Trends
The Preliminary Test Segment is Expected to Hold a Major Market Share in the Prostate-Specific Antigen Test Market
PSA screening has been more widely available, allowing for earlier detection and treatment, which has resulted in higher survival rates. PSA testing discovers previously undetected and tiny lesions that may or may not progress to a more advanced cancer stage. Most men over 50 and those at risk of acquiring the disease should get these tests since they help diagnose the disease before serious symptoms arise.
The increasing incidence of prostate cancer among men creates the need for advanced diagnostic products, thereby driving the market's growth. Prostate cancer is the most common cancer in American men. As per the American Cancer Society updates from January 2022, it is estimated that about 268,490 new cases of prostate cancer will be diagnosed in the United States in 2022.
Moreover, according to Globocan 2020, prostate cancer is the second most common type of cancer in the male population. As per the data published by the same source, the prevalence of prostate cancer was found to be 1,414,259, or 7.3% of the total cancer cases worldwide. As prostate cancer is more common in the elderly population, the rising aging population in the world is also expected to propel market growth. For instance, according to the World Population Aging Highlight of 2020 published by the United Nations, the global geriatric population (people age 65 years or more) was 727 million in 2020, and it is expected to reach 1.5 billion by the end of 2050. Thus, the increasing number of prostate cancer cases is expected to increase the demand for the preliminary test, thus, driving the segment's growth.
As a result of the aforementioned factors, the preliminary test segment is predicted to develop significantly throughout the forecast period.
North America is Expected to Hold a significant share in the market and is expected to do the Same in the Forecast Period.
North America is anticipated to hold a significant market share, owing to the factors such as the increasing prevalence of prostate cancer, coupled with the rising awareness and increasing research and development in this region. However, the current impact of the COVID-19 pandemic on cancer care in the United States has resulted in decreases and delays in identifying new cancers and the delivery of treatment. As per the research article published in October 2021, "Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors", significant decreases in screening for prostate cancer were observed in 2020 compared with 2019, with the most significant reduction occurring in April for prostate (56%) screenings. Thus, it is expected to increase the burden of prostate cancer in the region, in turn, it is expected to boost the growth of the market over the forecast period.
The market's expansion is further aided by favorable government backing in the United States. For example, Datar Cancer Genetics reported in February 2022 that its TriNetra-Prostate blood test, created to identify early-stage prostate cancer, has been designated a breakthrough device by the United States Food and Drug Administration. Paige Prostate, the first artificial intelligence (AI) based program developed to detect areas at risk of being malignant in prostate biopsy photos, was also approved by the United States Food and Drug Administration (FDA) in September 2021. This program allows pathologists to make a higher number of correct malignant tissue diagnoses, perhaps helping to address the growing number of cancer cases worldwide.
As a result of the aforementioned factors, the region is predicted to show significant growth during the forecast period.
Prostate Specific Antigen Test Industry Overview
The prostate-specific antigen test market is fragmented and competitive and consists of several major players. Some of the players operating in the market are Accuquik Test Kits, Bayer AG, Bio-Rad Laboratories, Inc., Beckman Coulter, Inc., Fujirebio (H.U. Group company), General Electric Company, Abcam PLC, Lomina AG, Laboratory Corporation of America Holdings, OPKO Health, Inc., Hanzhou Testsea biotechnology Co. LTD, and Proteomedix.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Prevalence of Prostate Cancer
- 4.2.2 Increasing Government Initiatives
- 4.2.3 Technological Advancements
- 4.3 Market Restraints
- 4.3.1 High Cost Of Diagnosis
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Test Type
- 5.1.1 Preliminary Tests
- 5.1.2 Confirmatory Tests
- 5.1.2.1 Pca3 Test
- 5.1.2.2 Trans-Rectal Ultrasound
- 5.1.2.3 Biopsy
- 5.2 Geography
- 5.2.1 North America
- 5.2.1.1 United States
- 5.2.1.2 Canada
- 5.2.1.3 Mexico
- 5.2.2 Europe
- 5.2.2.1 Germany
- 5.2.2.2 United Kingdom
- 5.2.2.3 France
- 5.2.2.4 Italy
- 5.2.2.5 Spain
- 5.2.2.6 Rest of Europe
- 5.2.3 Asia-Pacific
- 5.2.3.1 China
- 5.2.3.2 Japan
- 5.2.3.3 India
- 5.2.3.4 Australia
- 5.2.3.5 South Korea
- 5.2.3.6 Rest of Asia-Pacific
- 5.2.4 Middle-East and Africa
- 5.2.4.1 GCC
- 5.2.4.2 South Africa
- 5.2.4.3 Rest of Middle-East and Africa
- 5.2.5 South America
- 5.2.5.1 Brazil
- 5.2.5.2 Argentina
- 5.2.5.3 Rest of South America
- 5.2.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Accuquik Test Kits
- 6.1.2 Bayer AG
- 6.1.3 Bio-Rad Laboratories, Inc.
- 6.1.4 Beckman Coulter, Inc.
- 6.1.5 Fujirebio (H.U. Group company)
- 6.1.6 General Electric Company
- 6.1.7 Abcam plc.
- 6.1.8 Lomina AG.
- 6.1.9 Laboratory Corporation of America Holdings
- 6.1.10 OPKO Health, Inc.
- 6.1.11 Hanzhou Testsea biotechnology co., LTD.
- 6.1.12 Proteomedix

















