
|
시장보고서
상품코드
1444571
피부 치료제 : 시장 점유율 분석, 업계 동향과 통계, 성장 예측(2024-2029년)Dermatological Therapeutics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
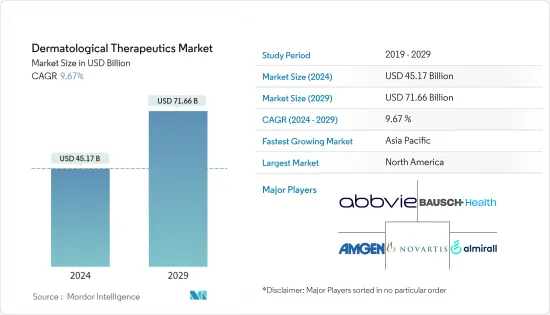
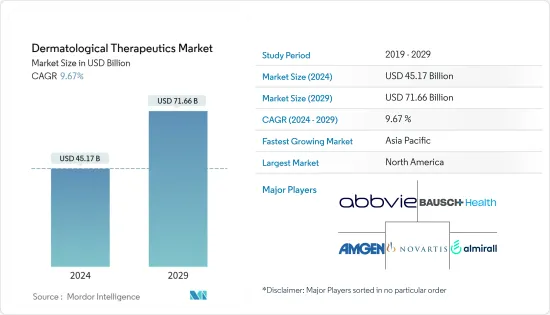
피부 치료제 시장 규모는 2024년에 451억 7,000만 달러로 추정되며, 2029년까지 716억 6,000만 달러에 달할 것으로 예측되고 있으며, 예측 기간(2024-2029년) 중 9.67%의 CAGR로 성장합니다.
전 세계에서 볼 때, COVID-19의 높은 감염률로 인해 전 세계 많은 국가들이 경제와 의료 시스템에 큰 부담을 겪었고, 현재도 겪고 있으며, COVID-19 팬데믹 기간 중 모든 의료 관련 기업의 제조 부문이 영향을 받았고, 모든 첨단 시장과 신흥 시장에서 교통이 큰 영향을 받았습니다. 모든 첨단시장과 신흥 시장에서 운송이 큰 영향을 받았습니다. 피부과 시장은 팬데믹 기간 중 몇 가지 간접적, 직접적 영향을 관찰했습니다. 예를 들어 2021년 1월 PubMed Central이 발표한 논문에 따르면 COVID-19가 대부분의 피부과 서비스에 악영향을 미쳐 만성 환자 진료 시간이 크게 감소했다는 연구 결과가 발표되었습니다. 따라서 전염병은 시장 성장에 큰 영향을 미쳤습니다. 그러나 전염병이 진정됨에 따라 조사 대상 시장은 예측 기간 중 안정적인 성장을 이룰 것으로 예상됩니다.
시장 성장을 가속하는 요인으로는 피부과 질환의 부담 증가, 질환의 진행과 병인에 대한 인식 수준 향상, 노인 인구 증가 등을 꼽을 수 있습니다. 고령화가 진행됨에 따라 피부과 진료는 앞으로 점점 더 많은 관심을 받게 될 것입니다. 노화에 따른 결합조직의 변화, 피부의 강도와 탄력성 감소, 피지선의 분비물 감소 등의 요인으로 인해 피부 관련 질환의 발병 위험이 증가합니다.
예를 들어 2022년에 발간된 '세계 인구 예측 2022 보고서'에 따르면 세계 인구에서 65세 이상 인구가 차지하는 비율은 2022년 10%에서 2050년 16%로 증가할 것으로 예상되며, 2050년에는 65세 인구가 5세 미만 어린이 인구의 두 배에 달할 것으로 예측됩니다. 12세 미만 아동 수의 2배 이상, 12세 미만 아동 수와 거의 같을 것으로 예측됩니다. 따라서 노인 인구 증가는 시장 성장을 가속할 것으로 예상됩니다.
피부질환은 사람들의 삶의 질에 심각한 영향을 미치고, 직장과 기타 장소에서 생산성을 떨어뜨리고 외모로 인한 차별을 유발합니다. 2022년 국제습진위원회가 발표한 2022년 아토피피부염 세계보고서에 따르면 2022년에는 약 2억 2,300만 명이 아토피피부염을 앓고 있으며, 그 중 약 4,300만 명은 1-4세였습니다. 이는 유아의 유병률이 놀라울 정도로 높다는 것을 보여줍니다. 아토피 피부염은 아이와 보호자에게 질병 부담이 될 뿐만 아니라 아이의 발달, 교육, 직업에도 악영향을 미칠 수 있습니다. 따라서 이러한 피부 질환의 높은 유병률과 부담 증가는 시장 성장을 가속할 것으로 예상됩니다.
피부 치료제 시장은 협업, 인수, 신제품 출시로 인해 상당한 성장 기회가 있을 것으로 예상됩니다. 예를 들어 2021년 12월 LEO Pharma Inc.는 미국 FDA가 18세 이상 성인의 중등도에서 중증의 아토피 피부염 치료제로 Adbly(트라로퀴누맙-ldrm)를 승인했다고 발표했습니다. 또는 이러한 치료법이 권장되지 않는 경우.
따라서 피부 질환의 부담 증가, 노인 인구 증가, 주요 시장 기업의 개발 증가는 예측 기간 중 시장을 주도할 것으로 예상됩니다. 그러나 특정 유형의 치료제의 심각한 부작용은 가까운 미래에 시장을 억제할 것으로 예상됩니다.
피부치료제 시장 동향
건선 부문은 예측 기간 중 상당한 시장 점유율을 유지할 것으로 예상됩니다.
건선은 피부 세포의 성장 주기를 가속화하는 만성 자가면역성 피부 질환입니다. 팔꿈치, 얼굴, 손바닥, 무릎, 두피, 허리, 발바닥에 두꺼운 붉은 피부와 은색 비늘 모양의 반점이 생깁니다. 가장 흔한 유형의 건선은 피부에 대한 잘못된 T 세포 공격으로 인해 발생하는 심상성 건선입니다. 건선 유병률 증가, 건선 치료제 개발 임상시험 증가, 주요 시장 기업의 개발 증가는 이 부문의 성장을 가속할 것으로 예상됩니다.
건선 유병률 증가가 이 부문을 이끄는 주요 요인입니다. 예를 들어 국립 건선 재단이 2022년 12월에 업데이트한 데이터에 따르면 2022년에는 전 세계에서 약 1억 2,500만 명이 건선을 앓고 있으며, 이는 전체 인구의 2-3%에 해당합니다. 이 정보원은 또한 2022년에는 800만 명 이상의 미국인이 건선을 앓고 있다고 밝혔습니다. 따라서 건선의 높은 유병률은 피부과 치료의 채택을 촉진할 것으로 예상됩니다. 건선은 종종 노인과 관련이 있으므로 노인 인구 증가도 부문별 성장을 가속할 것으로 예상됩니다.
Clinicaltrials.gov가 발표한 데이터에 따르면 2023년 2월 현재 전 세계에서 건선 치료를 위한 약 325건의 임상시험이 진행 중입니다. 향후 이 질환의 치료제로 승인될 것으로 예상되며, 이는 이 부문의 성장을 촉진할 것으로 예상됩니다.
신제품 출시, 시장 관계자들의 피부 치료제 시장 확대에 대한 관심, 제휴 및 인수합병이 이 분야를 견인할 것으로 예상됩니다. 예를 들어 2021년 12월 미국 FDA는 경증에서 중등도 판상 건선 성인 치료에 암젠의 의약품 오테즈라(Otezra)의 사용 확대를 승인했습니다.
따라서 건선 유병률 증가, 노인 인구 증가, 주요 기업의 개발 증가, 건선 임상시험 수 증가 등의 요인으로 인해 이 부문은 예측 기간 중 꾸준한 성장세를 보일 것으로 예상됩니다.
북미는 예측 기간 중 상당한 시장 점유율을 차지할 것으로 예상
북미는 의료비 증가, 피부 질환에 대한 인식 증가, 제약사의 강력한 존재감으로 인해 큰 시장 점유율을 유지할 것으로 예상됩니다.
피부과 질환의 유병률 증가는 이 지역 시장을 촉진할 것으로 예상되는 주요 요인입니다. 예를 들어 2022년 8월 미국 천식 및 알레르기 재단이 발표한 자료에 따르면 2022년 약 1,650만 명의 성인이 아토피 피부염(AD)을 앓고 있으며, 2022년에는 660만 명이 중등도에서 중증의 아토피 피부염을 앓고 있다고 보고했습니다. 이러한 피부과 질환은 이 지역에서 피부과 치료의 채택을 촉진할 것으로 예상됩니다.
노인 인구 증가도 시장의 주요 촉진요인입니다. 예를 들어 캐나다 통계청이 2022년 7월 발표한 자료에 따르면 캐나다의 65세 이상 인구는 약 7,330,605명으로 전체 인구의 18.8%를 차지합니다.
주요 지역 시장 기업의 신제품 출시도 시장 성장을 가속할 것으로 예상됩니다. 예를 들어 2021년 7월 Sol-Gel Technologies Ltd는 성인 및 소아 환자의 심상성 여드름 치료를 위한 최초의 독점 의약품 TWYNEO(트레티노인/벤조일 과산화물) 크림 0.1%/3%가 FDA의 승인을 받았다고 발표했습니다. 9세 이상. 마찬가지로 2021년 9월, 인사이트는 미국 FDA가 12세 이상의 비면역결핍 환자에서 경증에서 중등도 아토피피부염(AD)의 단기 및 비지속적 만성 치료제로 옵디젤라(룩소리티닙) 크림을 승인했다고 발표했습니다. 국소 처방 요법으로 질병이 적절히 조절되지 않거나 이러한 요법이 권장되지 않는 경우. 따라서 주요 시장 기업의 제품 출시 증가는 주로 북미 시장을 주도하고 있습니다.
따라서 피부과 질환의 유병률 증가, 노인 인구 증가, 주요 시장 기업의 개발 증가 등의 요인으로 인해 시장은 예측 기간 중 이 지역에서 꾸준한 성장을 이룰 것으로 예상됩니다.
피부치료제 산업 개요
피부치료제 시장은 적당히 세분화되어 있습니다. 성장 기회로 인해 피부 치료제 시장에 많은 신규 기업이 등장하고 있습니다. 주요 의약품의 향후 특허 만료로 인해 경쟁이 심화되고 있으며, 특히 제네릭 분야에서 시장 조사를 더욱 촉진하고 있습니다. 피부과 진단 및 치료 산업은 일부 제네릭 기업이 개발도상국에서 큰 시장 점유율을 차지하고 있으며 크게 성장할 것으로 예상됩니다. Amgen Inc., Bausch Health Companies Inc., Novartis AG, AbbVie Inc., Almirall SA 등이 시장 참여기업입니다.
기타 혜택
- 엑셀 형식의 시장 예측(ME) 시트
- 3개월간의 애널리스트 지원
목차
제1장 서론
- 조사의 전제조건과 시장의 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 촉진요인
- 증대하는 피부과 질환의 부담
- 질병의 진행과 병인에 관한 의식 레벨의 향상
- 고령자 인구의 증가
- 시장 억제요인
- 특정 유형의 치료제의 중증 부작용
- Porter's Five Forces 분석
- 신규 진출업체의 위협
- 구매자의 교섭력
- 공급 기업의 교섭력
- 대체품의 위협
- 경쟁 기업간 경쟁의 강도
제5장 시장 세분화
- 용도별
- 탈모증
- 헤르페스
- 건선
- 주사
- 아토피 피부염
- 기타 용도
- 약제 클래스별
- 항감염제
- 코르티코스테로이드
- 여드름 대책
- 칼시뉴린 저해제
- 레티노이드
- 기타 약제 클래스
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카공화국
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 개요
- Abbvie Inc.(Allergan PLC)
- Almirall SA
- Amgen Inc.
- Bausch Health Companies Inc.
- Galderma SA
- GlaxoSmithKline PLC
- Johnson &Johnson
- Novartis AG
- Pfizer Inc.
- Bristol-Myers Squibb Company
- LEO Pharma AS
- Eli Lilly and Company
- Sun Pharmaceuticals Ltd
- Aclaris Therapeutics Inc.
- Aurobindo Pharma Ltd
제7장 시장 기회와 향후 동향
KSA 24.03.18The Dermatological Therapeutics Market size is estimated at USD 45.17 billion in 2024, and is expected to reach USD 71.66 billion by 2029, growing at a CAGR of 9.67% during the forecast period (2024-2029).
Globally, due to the high transmission rate of COVID-19, many countries worldwide have suffered and are continuing to suffer a major burden on their economy and healthcare systems. The manufacturing units of all healthcare-based companies were affected, and the transport was impacted badly across all developed and emerging markets during the COVID-19 pandemic. The dermatology market observed some indirect and direct impacts during the pandemic. For instance, according to an article published by PubMed Central in January 2021, a study showed that the COVID-19 outbreak had an adverse effect on most dermatology services, with a significant reduction in consultation time spent for chronic patients. Thus, the pandemic had a significant impact on the market growth. However, as the pandemic has subsided, the studied market is expected to have stable growth during the forecast period.
Some factors driving the market's growth include the growing burden of dermatology diseases, increased awareness levels of disease progression and etiology, and a rise in the elderly population. With the rise in the aging population, dermatological care is likely to receive particular attention. As people age, the risk of developing skin-related disorders increases due to factors such as changes in the connective tissue, reduction in the skin's strength and elasticity, and reduction in secretions from sebaceous glands.
For instance, according to the World Population Prospects 2022 Report published in 2022, the share of the global population aged 65 years or above is projected to rise from 10% in 2022 to 16% in 2050. By 2050, the number of people aged 65 years or over worldwide is projected to be more than twice the number of children under age five and about the same as that of children under age 12. Thus, the rising geriatric population is expected to enhance market growth.
Skin diseases have seriously impacted people's quality of life, causing productivity loss at work and other places and discrimination due to disfigurement. According to the Global Report on Atopic Dermatitis 2022 published by the International Eczema Council in 2022, about 223 million people were living with atopic dermatitis in 2022, of which around 43 million were aged 1-4. This shows the strikingly high prevalence in young children. In addition to the disease burden for the children and their caregivers, atopic dermatitis can also negatively affect the children's development, education, and work. Thus, the high prevalence and the rising burden of such dermatological diseases are expected to boost the market's growth.
The dermatological therapeutics market is anticipated to witness a lucrative growth opportunity due to collaborations, acquisitions, and new product launches. For instance, in December 2021, LEO Pharma Inc. announced that the US FDA approved Adbry (tralokinumab-ldrm) for the treatment of moderate-to-severe atopic dermatitis in adults 18 years or older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
Thus, the increasing burden of dermatological diseases, the rising geriatric population, and the increasing developments by key market players are expected to drive the market over the forecast period. However, serious side effects of certain classes of therapeutic drugs are expected to restrain the market in the near future.
Dermatological Therapeutics Market Trends
Psoriasis Segment is Expected to Hold a Significant Market Share Over the Forecast Period
Psoriasis is a chronic autoimmune skin disease that speeds up the growth cycle of skin cells. It causes patches of thick red skin and silvery scales on the elbows, face, palms, knees, scalp, lower back, and soles of feet. The most common type of psoriasis is plaque psoriasis, and misguided T-cell attacks on the skin cause it. The rising prevalence of psoriasis, the increasing number of clinical trials for psoriasis drug development, and the rising developments by major market players are expected to boost the segment's growth.
The rising prevalence of psoriasis is a major factor driving the segment. For instance, according to the data updated by the National Psoriasis Foundation in December 2022, around 125 million people worldwide had psoriasis in 2022, almost equal to 2-3% of the total population. The source also stated that more than 8 million Americans had psoriasis in 2022. Thus, the high prevalence of psoriasis is expected to boost the adoption of dermatological therapeutics. As psoriasis is often associated with the elderly, the rising geriatric population is also expected to drive segmental growth.
As per the data published by clinicaltrials.gov, in February 2023, globally, approximately 325 ongoing clinical trials were being conducted for the treatment of psoriasis. These are expected to receive approvals for the treatment of the disease in the future, thus driving the segment's growth.
The new product launches, the focus of market players in expanding their market for dermatology therapeutics, and collaborations and acquisitions are anticipated to boost the segment. For instance, in December 2021, the US FDA approved the expanded use of Amgen Inc.'s drug Otezla to treat adults with mild to moderate plaque psoriasis.
Thus, due to factors such as the rising prevalence of psoriasis, the rising geriatric population, the increasing developments by key players, and the rising number of psoriasis clinical trials, the segment is expected to witness steady growth over the forecast period.
North America is Expected to Have a Significant Market Share Over the Forecast Period
North America is expected to hold a significant market share due to increasing healthcare expenditure, growing awareness about skin diseases, and the strong presence of pharmaceutical companies.
The rising prevalence of dermatological diseases is a major factor that is expected to boost the market in the region. For instance, according to the data published by the Asthma and Allergy Foundation of America in August 2022, around 16.5 million adults had atopic dermatitis (AD), with 6.6 million reporting moderate-to-severe symptoms in 2022. Thus, the high prevalence of such dermatological diseases is expected to boost the adoption of dermatological therapeutics in the region.
The rising geriatric population is also a major driver of the market. For instance, according to the data published by Statistics Canada in July 2022, around 7,330,605 people are 65 years of age or older in Canada, accounting for 18.8% of the total population.
The new product launches by key regional market players are also anticipated to boost the market's growth. For instance, in July 2021, Sol-Gel Technologies Ltd announced that the FDA approved its first proprietary drug product, TWYNEO (tretinoin/benzoyl peroxide) cream, 0.1%/3%, indicated for the treatment of acne vulgaris in adults and pediatric patients nine years of age and older. Similarly, in September 2021, Incyte announced that the US FDA approved Opzelura (ruxolitinib) cream for the short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis (AD) in non-immunocompromised patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies, or when those therapies are not advisable. Thus, the rising product launches by key market players are mainly driving the North American market.
Thus, due to factors such as the rising prevalence of dermatological diseases, the rising geriatric population, and the increasing developments by key market players, the market is expected to witness steady growth in the region over the forecast period.
Dermatological Therapeutics Industry Overview
The dermatological therapeutics market is moderately fragmented. Due to the growth opportunities, many new players are emerging in the dermatological therapeutics market. Forthcoming patent expiries of major drugs are leading to increased competition, further driving the market studied, especially in the generic sector. The dermatological diagnostics and therapeutics industry is expected to grow tremendously, with several generic players controlling significant market share in the developing regions. Some of the market players include Amgen Inc., Bausch Health Companies Inc., Novartis AG, AbbVie Inc., and Almirall SA.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Burden of Dermatology Diseases
- 4.2.2 Increasing Awareness Levels of Disease Progression and Etiology
- 4.2.3 Increasing Elderly Population
- 4.3 Market Restraints
- 4.3.1 Serious Side Effects for Certain Classes of Therapeutic Drugs
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Application
- 5.1.1 Alopecia
- 5.1.2 Herpes
- 5.1.3 Psoriasis
- 5.1.4 Rosacea
- 5.1.5 Atopic Dermatitis
- 5.1.6 Other Applications
- 5.2 By Drug Class
- 5.2.1 Anti-infectives
- 5.2.2 Corticosteroids
- 5.2.3 Anti-acne
- 5.2.4 Calcineurin Inhibitors
- 5.2.5 Retinoids
- 5.2.6 Other Drug Classes
- 5.3 By Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle East and Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Abbvie Inc. (Allergan PLC)
- 6.1.2 Almirall SA
- 6.1.3 Amgen Inc.
- 6.1.4 Bausch Health Companies Inc.
- 6.1.5 Galderma SA
- 6.1.6 GlaxoSmithKline PLC
- 6.1.7 Johnson & Johnson
- 6.1.8 Novartis AG
- 6.1.9 Pfizer Inc.
- 6.1.10 Bristol-Myers Squibb Company
- 6.1.11 LEO Pharma AS
- 6.1.12 Eli Lilly and Company
- 6.1.13 Sun Pharmaceuticals Ltd
- 6.1.14 Aclaris Therapeutics Inc.
- 6.1.15 Aurobindo Pharma Ltd

















