
|
시장보고서
상품코드
1521712
플라스미드 DNA 수탁제조 : 시장 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Plasmid DNA Contract Manufacturing - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
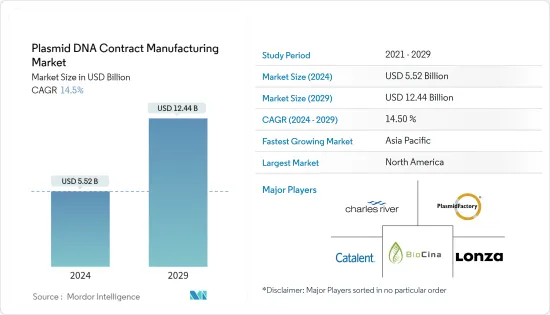
플라스미드 DNA 수탁제조(Plasmid DNA Contract Manufacturing) 시장 규모는 2024년 55억 2,000만 달러로 추정되고, 2029년 124억 4,000만 달러에 이를 것으로 예측되며, 예측 기간 중(2024-2029년) CAGR은 14.5%로 추이하며 성장 할 것으로 예측됩니다.

질병 유병률 증가, 조사 연구에 대한 투자, 유전자 치료의 인기가 시장 성장의 주요 요인입니다. 유전자 치료의 인기가 점점 증가하고 있기 때문에 유전자 치료 과정에서 플라스미드의 중요한 역할은 플라스미드 DNA 위탁 생산에 대한 수요를 증가시키고 있습니다. 플라스미드는 치료 유전자를 표적 세포에 도입하기 위한 캐리어로서 역할을 합니다. 더 많은 유전자 치료가 임상시험을 거쳐 상업 생산으로 진행됨에 따라 플라스미드 DNA의 대규모 고품질 생산에 대한 요구가 커지고 있습니다. 예를 들어, 2023년 8월, Charles River는 공급 부족을 해결하고 세포 및 유전자 치료 산업 증가 수요를 충족시키기 위해 세계적인 수준의 HQ 플라스미드 제조 센터를 설립했습니다.
만성 질환에서 감염의 위협에 이르기까지 다양한 건강 문제를 특징으로 하는 질병 부담 증가는 플라스미드 DNA 수탁제조 서비스 수요를 증가시키고 있습니다. 영국 심장재단의 2024년 1월 잉글랜드 팩트 시트에 따르면 2022년에는 영국에서 약 760만 명이 심혈관 질환을 앓고 있었습니다. 같은 출처에 따르면 2022년에는 세계 약 6억 2,000만 명이 심장·순환기 질환(심혈관 질환)을 앓고 있습니다. 이 수는 라이프 스타일 변화, 노화, 인구 증가로 인해 계속 증가하고 있습니다. 이와 같이 만성질환의 유병률이 증가함에 따라 신규 치료와 치료에 대한 수요도 증가하고 있습니다. 플라스미드 DNA의 수탁제조는 만성 질환에 대한 고도의 치료법을 개발하는 생명 공학 기업과 제약 기업의 요구에 부응함으로써 이 혜택을 받고 있습니다.
WHO가 2023년 3월에 발표한 보고서에 따르면, 2022년 말까지 약 3,900만 명이 HIV 감염자가 되고, 그 중에는 150만 명의 유아도 포함되어 있습니다. 또한 HIV와 함께 사는 2,980만 명이 세계에서 항레트로바이러스 치료를 받고 있습니다. 플라스미드 DNA는 유전자 요법과 백신 개발에 매우 중요하며 만성 질환 치료에 견인 역할을 합니다. 유전자 치료는 종종 치료 유전자를 세포 내로 도입하기 위해 플라스미드 DNA를 이용하며, 만성 질환과 관련된 유전적 장애를 대상으로 합니다. 그러므로 만성질환 부담 증가는 예측 기간 동안 시장 성장을 뒷받침할 것으로 예상됩니다.
이 시장 진출 기업은 플라스미드 DNA 수요 증가에 대응하기 위해 지속적으로 사업을 확대하고 있습니다. 각 회사는 공정 개발 및 최적화, 플라스미드 설계, 플라스미드 공학, 플라스미드 구축, GMP 플라스미드 제조를 포함한 다양한 플라스미드 서비스를 제공함으로써 완전히 통합된 원스톱 숍으로서 역할을 할 수 있도록 정력적으로 능력을 높이고 있습니다.
예를 들어, Catalent는 2023년 1월 벨기에 대한 고세리스에 있는 이 회사의 유럽 세포 치료 센터 오브 엑설런스 내에 새로운 상업용 등급 플라스미드 DNA(pDNA) 생산 시설을 개설했습니다. 이 시설은 임상 및 상업 단계공급을 위해 CGMP 등급 pDNA를 생산하기 위해 여러 클린 룸에 걸쳐 12,000 평방 피트(1,100 평방미터)의 개발 및 제조 공간을 가지고 있습니다.
이와 같이, 세포 및 유전자 치료에 대한 수요 증가와 시장 관계자의 전략적 활동이 예측 기간 중 시장 성장을 뒷받침할 것으로 예상됩니다. 그러나 특히 신흥 국가에서는 제조를 위한 첨단 인프라가 필요하며, 수탁제조와 관련된 품질 문제가 예측 기간 동안 시장 성장을 억제할 것으로 예상됩니다.
플라스미드 DNA 수탁제조 시장 동향
세포 및 유전자 치료 부문은 예측 기간 동안 상당한 성장이 예상
세포 및 유전자 치료 시장은 세포·유전자 기반의 플라스미드 DNA 제조 서비스를 추가하는 개발 업무 수탁 기관(CDO) 증가에 의해 확대가 전망됩니다. 예를 들어, 2022년 10월, Ray Therapeutics와 개발·제조 수탁기관(CDMO)의 Forge Biologics는 임상 단계의 플라스미드 DNA를 제조하는 계약을 연장하고, Ray Therapeutics의 획기적인 옵토제네틱스 유전자 치료 프로그램을 지원합니다. 또한 2023년 10월, BioCina Pty Ltd는 GenomeFrontier Therapeutics AU Pty Ltd와 새로운 파트너십을 체결하여 CAR-T 세포 치료 제품을 지원하기 위한 미니서클 DNA 및 플라스미드 DNA의 공정 개발과 GMP 제조를 GenomeFrontier에 지원한다고 발표했습니다.
플라스미드 DNA 플랫폼의 기술적 진보와 수탁제조를 통한 첨단 기술의 채택은 아웃소싱 수요를 증가시키고 이 부문의 성장을 뒷받침할 것으로 예상됩니다. 예를 들어, 2023년 1월, Charles River Laboratories International Inc.는 회사의 개발 및 제조 위탁(CDMO)과 생물학적 제제 시험 경험에서 확립된 eXpDNA 플라스미드 플랫폼을 발표했습니다. 이 플랫폼은 플라스미드의 개발·제조 기간을 대폭 단축함과 동시에 세포 및 유전자 치료나 백신 개발자의 개발 기간을 합리화하여 제품의 품질과 일관성에 중점을 둔 것입니다.
유전자 치료 개발 기업은 유전자 치료에서 플라스미드 DNA 수요 증가에 대응하기 위해 전문 지식, 인프라, 규제 컴플라이언스를 활용하기 위해 이러한 전문 제조 시설과 협력하는 경우가 많습니다. 이러한 아웃소싱을 통해 유전자 치료 기업은 상업적 규모의 플라스미드 DNA의 효율적이고 신뢰성 있는 생산을 보장하면서 치료의 임상 및 규제 측면에 집중할 수 있습니다. 2023년 6월, INADcure 재단과 Charles River Laboratories는 소아 신경 축삭성 이영양증을 표적으로 하는 유전자 치료의 I/II 상 임상시험을 위한 고품질 플라스미드 DNA(pDNA) 생산에 협력했습니다.
따라서, 세포 및 유전자 치료에 대한 수요 증가와 시장 진출기업에 의한 전략적 활동이 예측 기간 동안 이 부문의 성장에 기여할 것으로 예상됩니다.
예측기간 중 북미가 시장에서 큰 점유율을 차지할 전망
북미에서는 확립된 연구시설, 세포치료를 위한 연구개발투자 증가, 만성질환 부담 증가 등 요인으로 인해 플라스미드 DNA 수탁제조시장 성장이 전망되고 있습니다. 플라스미드 DNA 수탁제조 시장 성장은 제품상 시수 증가, 진행 중인 다수의 임상시험, 자가면역질환, 암, 감염증 치료에 대한 세포 치료의 응용 가능성 등에 기인하고 있습니다.
세포 및 유전자 치료에 대한 수요 증가는 예측 기간 중 시장 성장을 뒷받침할 것으로 예상됩니다. 2023년 10월 현재, 북미에서는 세포 요법에 초점을 맞춘 581건 이상의 임상시험이 실시되어, 다양한 질병의 치료에 대한 이러한 치료의 가능성이 해석되고 있습니다. 그러므로, 임상 개발에서 연구 증가는 예측 기간에 걸쳐 플라스미드 DNA 수탁제조 시장을 밀어올릴 것으로 예상됩니다.
시장 진출기업에 의한 아웃소싱 및 전략적 활동 동향 증가는 예측 기간 동안 시장 성장에 기여할 것으로 예상됩니다. 예를 들어, 2022년 10월, Forge Biologics는 9,000만 달러의 시리즈 C 자금 조달 라운드를 완료한 후 AAV 클라이언트를 지원하기 위해 플라스미드 DNA(pDNA) 제조 서비스를 시작했습니다. 마찬가지로 2022년 5월 Avantor는 Cytovance Biologics(플라스미드 DNA에 특화된 연구개발·제조 수탁기관)와 바이러스 벡터와 RNA 기반 백신 및 치료용 연구 등급과 GMP 등급 플라스미드를 제조하는 계약 를 체결했습니다.
따라서, 세포치료와 유전자치료를 위한 임상연구수 증가, 플라스미드 DNA 제조시설의 확대, 시장 진출기업에 의한 전략적 제휴는 예측기간에 걸쳐 시장을 밀어올릴 것으로 예상됩니다.
플라스미드 DNA 수탁제조 산업 개요
플라스미드 DNA 수탁제조 시장은 적당한 경쟁 관계에 있으며, 서비스 확대, 제휴, 공동 연구, M&A 등 전략적 활동에 종사하는 대기업과 중소기업 모두가 존재합니다. PlasmidFactory GmbH &Co.KG, Lonza, BioCina, Charles River Laboratories, Catalent Inc. 등이 이 시장에서 주목할 만한 주요 기업입니다.
기타 혜택 :
- 엑셀 형식 시장 예측(ME) 시트
- 애널리스트에 의한 3개월간의 지원
목차
제1장 서론
- 조사 전제 조건과 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 질병 유병률 증가
- 조사연구 투자확대
- 유전자 치료의 인기 증가
- 시장 성장 억제요인
- 일부 신흥 국가에서 제조를 위한 첨단 인프라 부족
- 수탁제조에 따른 품질 문제
- Porter's Five Forces 분석
- 공급기업의 협상력
- 구매자/소비자의 협상력
- 신규 진입업자의 위협
- 대체품의 위협
- 경쟁 기업간 경쟁 관계 강도
제5장 시장 세분화(시장 규모-달러)
- 용도별
- 세포 및 유전자 치료
- 면역치료
- 기타
- 치료 영역별
- 암
- 감염증
- 자가면역질환
- 순환기 질환
- 기타
- 최종 사용자별
- 제약·바이오테크놀러지 기업
- 연구기관
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 영국
- 독일
- 프랑스
- 스페인
- 이탈리아
- 기타 유럽
- 아시아 태평양
- 인도
- 일본
- 중국
- 호주
- 한국
- 기타 아시아 태평양
- 중동 및 아프리카
- GCC 국가
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 프로파일
- Charles River Laboratories
- VGXI Inc.
- PlasmidFactory GmbH & Co. KG
- Boehringer Ingelheim
- BioCina
- TriLink Biotechnologies
- Esco Aster PTE. LTD
- Thermo Fisher Scientific Inc.
- VIVE biotech
- Lonza
제7장 시장 기회 및 향후 동향
LYJThe Plasmid DNA Contract Manufacturing Market size is estimated at USD 5.52 billion in 2024, and is expected to reach USD 12.44 billion by 2029, growing at a CAGR of 14.5% during the forecast period (2024-2029).
The growing disease prevalence, investment in research studies, and popularity of gene therapy are the key factors driving the growth of the market. The ever-increasing popularity of gene therapy drives the demand for plasmid DNA contract manufacturing due to the essential role of plasmids in the gene therapy process. Plasmids serve as carriers for introducing therapeutic genes into target cells. As more gene therapy treatments advance through clinical trials and into commercial production, there is an increasing need for large-scale, high-quality production of plasmid DNA. For instance, in August 2023, Charles River established a world-class HQ plasmid manufacturing center of excellence to combat supply scarcity and fulfill the escalating demands of the cell and gene therapy industry.
The increasing disease burden, characterized by diverse health challenges ranging from chronic diseases to infectious threats, increases the demand for plasmid DNA contract manufacturing services. According to the British Heart Foundation's January 2024 England Factsheet, approximately 7.6 million individuals in England had cardiovascular diseases in 2022. As per the same source, around 620 million people live with heart and circulatory diseases (cardiovascular diseases) across the world in 2022; this number has been rising since then due to changing lifestyles, aging, and the growing population. Thus, with the rise in the prevalence of chronic diseases, there is a corresponding increase in demand for novel therapeutics and treatments. Plasmid DNA contract manufacturing is poised to benefit from this by meeting the requirements of biotech and pharmaceutical companies that are developing advanced therapies for chronic conditions.
As per the report published by WHO in March 2023, approximately 39.0 million people were living with HIV by the end of 2022, including 1.5 million children. Moreover, 29.8 million people living with HIV were receiving antiretroviral therapy globally. Plasmid DNA is crucial in gene therapy and vaccine development, gaining traction in treating chronic diseases. Gene therapies often utilize plasmid DNA to deliver therapeutic genes into cells, targeting genetic disorders associated with chronic conditions. Hence, the growing burden of chronic diseases is expected to boost the market's growth over the forecast period.
The players in this market are continuously expanding their operations to accommodate the growing demand for plasmid DNA. They are vigorously advancing their capabilities to serve as a fully integrated one-stop-shop by offering various plasmid services, including process development and optimization, plasmid design, plasmid engineering, plasmid construction, and GMP plasmid manufacturing.
For instance, in January 2023, Catalent inaugurated a novel commercial-grade plasmid DNA (pDNA) production facility within its European Center of Excellence for Cell Therapies in Gosselies, Belgium. The facility has 12,000 square feet (1,100 m2) of development and manufacturing space across multiple cleanrooms for CGMP-grade pDNA production for clinical and commercial-phase supply.
Thus, the growing demand for cell and gene therapy and strategic activities from the market players is expected to boost the market's growth over the forecast period. However, the need for advanced infrastructure for manufacturing in particular developing countries and quality issues associated with contract manufacturing are anticipated to restrain the market growth over the forecast period.
Plasmid DNA Contract Manufacturing Market Trends
The Cell & Gene Therapy Segment is Expected to Grow Significantly Over the Forecast Period
The cell & gene therapy market is anticipated to expand due to the growing number of contract development organizations (CDOs) adding cell and gene-based plasmid DNA manufacturing services to their offerings. For instance, in October 2022, Ray Therapeutics and contract development and manufacturing organization (CDMO) Forge Biologics extended their agreement to manufacture clinical-stage plasmid DNA, supporting Ray Therapeutics' groundbreaking optogenetics gene therapy program. Moreover, in October 2023, BioCina Pty Ltd announced a new partnership with GenomeFrontier Therapeutics AU Pty Ltd to support GenomeFrontier with process development and GMP manufacturing for Minicircle DNA and Plasmid DNA to support the CAR-T cell therapy product.
The technological advancement of the plasmid DNA platform and the introduction of advanced technologies by contract manufacturing are expected to increase the outsourcing demand, thereby boosting the segment's growth. For instance, in January 2023, Charles River Laboratories International Inc. launched its eXpDNA plasmid platform, established from the company's contract development and manufacturing (CDMO) and biologics testing experience. The platform significantly reduces plasmid development and production timelines while streamlining the development journey for cell and gene therapy and vaccine developers, focusing on product quality and consistency.
Gene therapy developers often collaborate with these specialized manufacturing facilities to leverage their expertise, infrastructure, and regulatory compliance to meet the increasing demand for plasmid DNA in gene therapy treatments. This outsourcing allows gene therapy companies to focus on the clinical and regulatory aspects of their treatments while ensuring the efficient and reliable production of plasmid DNA at a commercial scale. In June 2023, INADcure Foundation and Charles River Laboratories collaborated on the production of high-quality plasmid DNA (pDNA) for Phase I/II clinical trials of a gene therapy targeting Infantile Neuroaxonal Dystrophy.
Hence, the growing demand for cell and gene therapy and strategic activities by the market players are expected to contribute to the segment's growth over the forecast period.
North America is Expected to Hold a Significant Share of the Market Over the Forecast Period
In North America, the plasmid DNA contract manufacturing market is expected to grow owing to factors such as established research facilities, increasing investment in R&D for cell therapy, and the growing burden of chronic diseases. The growth of the plasmid DNA contract manufacturing market can be attributed to the increasing number of product launches, the high number of ongoing clinical trials, and the potential application of cell therapies in the treatment of autoimmune diseases, cancer, and infectious diseases.
The growing demand for cell and gene therapy is expected to boost the market's growth over the forecast period. As of October 2023, more than 581 clinical trials focusing on cell therapies were conducted to interpret the potential of these therapies for treating various disease indications in North America. Therefore, the increasing research in clinical development is expected to boost the plasmid DNA contract manufacturing market over the forecast period.
The growing trend of outsourcing and strategic activities by the market players is expected to contribute to the market's growth over the forecast period. For instance, in October 2022, Forge Biologics launched plasmid DNA (pDNA) manufacturing services to support its AAV clients after closing a USD 90 million Series C funding round. Similarly, in May 2022, Avantor signed an agreement with Cytovance Biologics (a contract development and manufacturing organization specializing in plasmid DNA) in order to manufacture research-grade and GMP-grade plasmids for viral vectors and m RNA-based vaccines and therapeutics.
Hence, the increase in the number of clinical research for cell and gene therapy, expansion of plasmid DNA manufacturing facilities, and strategic collaboration by the market players are expected to boost the market over the forecast period.
Plasmid DNA Contract Manufacturing Industry Overview
The plasmid DNA contract manufacturing market is moderately competitive, with the presence of both small and large players who are involved in strategic activities such as the expansion of services, partnerships, collaborations, and mergers and acquisitions. PlasmidFactory GmbH & Co. KG, Lonza, BioCina, Charles River Laboratories, and Catalent Inc. are among the notable key players in this market.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definitions
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Disease Prevalence
- 4.2.2 Growing Investments in Research Studies
- 4.2.3 Growing Popularity of Gene Therapy
- 4.3 Market Restraints
- 4.3.1 Lack of Advanced Infrastructure For Manufacturing in Certain Developing Countries
- 4.3.2 Quality Issues Associated With Contract Manufacturing
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Bargaining Power of Suppliers
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Threat of New Entrants
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Application
- 5.1.1 Cell & Gene Therapy
- 5.1.2 Immunotherapy
- 5.1.3 Others
- 5.2 By Therapeutic Area
- 5.2.1 Cancer
- 5.2.2 Infectious Diseases
- 5.2.3 Autoimmune Diseases
- 5.2.4 Cardiovascular Diseases
- 5.2.5 Others
- 5.3 By End User
- 5.3.1 Pharmaceutical and Biotechnology Companies
- 5.3.2 Research Institutes
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 United Kingdom
- 5.4.2.2 Germany
- 5.4.2.3 France
- 5.4.2.4 Spain
- 5.4.2.5 Italy
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 India
- 5.4.3.2 Japan
- 5.4.3.3 China
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of the Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Charles River Laboratories
- 6.1.2 VGXI Inc.
- 6.1.3 PlasmidFactory GmbH & Co. KG
- 6.1.4 Boehringer Ingelheim
- 6.1.5 BioCina
- 6.1.6 TriLink Biotechnologies
- 6.1.7 Esco Aster PTE. LTD
- 6.1.8 Thermo Fisher Scientific Inc.
- 6.1.9 VIVE biotech
- 6.1.10 Lonza

















