
|
시장보고서
상품코드
1521715
저분자 이노베이터 CDMO 시장 : 시장 점유율 분석, 업계 동향 및 통계, 성장 예측(2024-2029년)Small Molecules Innovator Contract Development And Manufacturing Organization - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
저분자 이노베이터 CDMO 시장 규모는 2024년 484억 달러로 추정되고, 2029년에는 699억 달러에 이를 전망이며, 예측기간 중(2024-2029년) CAGR은 6.60%로 성장할 것으로 예측됩니다.

시장을 견인하는 것은 저분자 의약품에 대한 수요 증가, 저분자 의약품의 파이프라인의 충실, 만성 질환의 부담 증가, 의약품 연구개발 투자 증가입니다. 저분자 의약품 파이프라인이 충실하다는 것은 제약 기업과 생명공학 기업이 다양한 개발 단계에 있는 화합물을 더 많이 보유하고 있음을 의미합니다. 따라서 제제 개발, 공정 최적화, 제조 등 CDMO가 제공하는 전문 서비스에 대한 요구가 커지고 있습니다. 예를 들어, 2023년 2월에 발표된 바이오 산업 분석 보고서에 따르면, 2022년 시점에 47개의 저분자 신약(NCE)이 임상 파이프라인에 있습니다. 전통적인 전신용 저분자 항생제는 97%를 차지하였고 국소용 저분자는 저분자 파이프라인 NCEs의 3%를 차지하였습니다.
마찬가지로 Clinicaltrials.gov에 따르면 2024년 3월 현재 제2상 시험 중 저분자 의약품은 64개 품목이고 제III상 시험 중 저분자 의약품은 3개 품목입니다. 따라서 파이프라인의 성장은 다양한 치료 영역에 걸쳐 있으며, 여러 병리학에 대응하는 경우가 많습니다. 이러한 의약품 개발의 다양화는 다양한 부문에 익숙한 CDMO에 대한 수요를 높여 시장을 더욱 활성화시킵니다.
제약기업이 저분자 의약품의 연구개발(R&D)에 많은 투자를 하고 있기 때문에 개발제조수탁기관(CDMO)이 제공하는 서비스에 대한 수요가 높아지고 있습니다. 이러한 조직은 신약 후보를 발견 단계부터 임상시험에 이르기 위해 전문적인 전문 지식과 인프라가 필요합니다. 예를 들어 캠브렉스는 2022년 9월 저분자 의약품(API) 제조를 위한 노스캐롤라이나주 하이포인트 시설에 대한 3,000만 달러의 투자 초기 단계를 완료했습니다. 또한, 저분자 의약품 개발을 위한 전략적 제휴는 예측 기간 동안 시장 성장을 가속할 것으로 예상됩니다. 예를 들어, 2022년 1월, Sanofi와 Exscientia는 실제 환자 샘플을 활용한 Exscientia의 AI 주도형 플랫폼을 활용하여 종양학과 면역학에 걸친 최대 15개의 신규 저분자 화합물 후보를 개발할 목적으로, 전략적 연구 제휴와 라이선스 계약을 체결했습니다.
따라서 저분자 의약품의 강력한 파이프라인, 저분자 개발 투자 증가, 시장 진출기업의 주요 전략 채택 등이 부문의 성장을 가속할 것으로 예상됩니다. 그러나 엄격한 정부 규제 및 아웃소싱과 관련된 컴플라이언스 문제는 예측 기간 동안 시장 성장을 억제할 것으로 예상됩니다.
저분자 이노베이터 CDMO 시장 동향
신경 부문은 2024년부터 2029년까지 큰 점유율을 차지할 전망
저분자 이노베이터 CDMO 시장에서의 신경부문은 신경질환에 대한 인식과 이해의 높아짐에 의해 알츠하이머병, 파킨슨병, 간질, 기타 다양한 신경변성 질환 등의 질환을 대상으로 한 의약품의 개발에 중점을 두게 되어 현저한 성장을 이루고 있습니다.
예를 들어, Biogen은 2023년 7월 저분자 파이프라인 의약품인 즈라놀론(GABAA PAM)-대우울증 장애(MDD)의 3상 임상시험, BIIB131(플라스미노겐 활성화 인자)-급성 허 혈성 뇌졸중의 2상 임상시험을 발표했습니다. 마찬가지로 UCB SA는 신경 질환에 대한 4가지 혁신적인 저분자 의약품 파이프라인을 가지고 있다고 보고합니다. 그 중에는 펜플루라민(5-HT 효능제), 독세시틴과 독시리부티민(MT1621, 뉴클레오시드 요법), 민자솔민(a-syn 미스폴딩 억제제), STACCATO 알프라졸람(벤조디아제핀)이 포함되어 다양한 개발 단계에 있습니다. 이러한 저분자신약의 방대한 파이프라인은 공정개발, 분석시험, 제조를 위한 CDMO에 큰 수요를 창출할 것으로 예상되며, 2024년부터 2029년까지 부문 성장에 기여할 것으로 기대되고 있습니다.
파킨슨병, 근위축성 측색경화증(ALS), 헌팅턴 무도병, 알츠하이머병 등 중추신경계 질환 증가는 이러한 질환의 의약품 개발에는 다양한 임상시험 지원과 규제 당국의 권고 등이 필요하기 때문에 CRO 서비스 수요를 촉진하고 있습니다. 예를 들어 Alzheimer's Disease Facts and Figures Annual Report 2023이 발표한 데이터에 따르면 600만 명 이상의 미국인이 알츠하이머병을 앓고 있으며, 이 수치는 30년 이내에 거의 1,300만 명으로 증가할 것으로 예측됩니다.
또한 위의 출처에 따르면 2023년 미국은 알츠하이머병 및 기타 치매의 치료 및 관리에 3,450억 달러를 보냈습니다. 2050년까지 이러한 비용은 미화 1조 달러 가까이 증가할 수 있습니다. 따라서 알츠하이머 병의 높은 부담은 혁신적이고 효과적인 치료법에 대한 수요를 창출하고 연구 시장의 성장을 이끌고 있습니다.
게다가 세계보건기구(WHO)가 2023년 3월에 갱신한 데이터에 따르면 전 세계적으로 약 5,500만명이 치매를 앓고 있으며, 매년 1,000만 명 가까이 새롭게 치매를 발병하고 있습니다. 게다가 2023년 9월에 갱신된 Atlas of Multiple Sclerosis의 데이터에 따르면 2023년에는 세계에서 290만 명이 다발성 경화증을 앓고 있으며 Parkinson's Foundation이 발표한 데이터에 따르면 1,000만 명이 파킨슨병을 앓고 있습니다. 이러한 중추신경계 질환의 부담 증가는 2024년부터 2029년까지 중추신경계 치료 수요와 중요한 연구활동을 촉진할 것으로 예상됩니다.
제약 기업과 생명 공학 기업은 서비스 확대에 주력하는 자세를 강화하고 있습니다. 예를 들어, 2023년 9월, Cellectricon은 신경정신의료, 간질, 신경퇴행과 같은 치료 영역에서 신약을 가속화하기 위한 새로운 Neuroplasticity Services 모듈을 출시하여 신경과학 위탁 연구 포트폴리오를 확대했습니다.
따라서 연구개발 활동 증가, 강력한 파이프라인의 존재, 시장 진출기업에 의한 전략적 활동이 2024년부터 2029년까지 시장 성장에 기여할 것으로 예상됩니다.
북미가 2024년부터 2029년까지 큰 시장 점유율을 차지할 전망
북미, 특히 미국은 확립된 견고한 제약 산업의 본거지입니다. 이 지역에는 창약과 의약품 개발에 적극적으로 참여하는 혁신적인 제약 기업과 생명 공학 기업이 집중되어 있습니다. 연구개발에 대한 높은 투자액, 주요 시장 진입기업의 견고한 발판, 미국 국립위생연구소(National Institute of Health)에 의한 신규 치료 개발을 위한 보조금 증가도 이 나라 시장 성장에 기여합니다.
제약 및 바이오테크놀러지 업계에 의한 저분자 의약품 개발에 대한 투자가 증가하고 있는 것도 시장 성장에 기여할 것으로 예상됩니다. 예를 들어, 2023년 5월 PharmEnable은 임상 요구가 높은 질병 영역에 대한 차세대 저분자 의약품을 개발하기 위해 750만 달러의 프리시리즈 A 투자 라운드를 종료했다고 발표했습니다. 게다가, 시장 진출기업은 서비스를 확대하기 위해 노력하고 있으며, 협업이 시장 성장을 가속할 가능성이 높습니다. 예를 들어 CDMO의 Phlow는 2023년 4월 시리즈 B 증자로 3,600만 달러를 조달했다고 발표했습니다. Phlow는 이 자금을 'cdmoX'라고 불리는 CDMO 프로그램의 확대를 포함한 상업적 제공의 확대에 충당한다고 말합니다. 게다가 2022년 7월에는 제약 및 바이오의약품 업계에서 저분자 및 고분자 바이오 분석 서비스를 제공하는 Alliance Pharma가 LGC로부터 Drug Development Solutions(DDS)의 인수를 완료했습니다. Ampersand Capital Partners와 KKR & Co.Ltd.에서 인수를 완료했습니다.
따라서 시장 진출기업에 의한 투자와 전략적 활동 증가는 2024년부터 2029년까지 이 지역 시장을 밀어올릴 것으로 예상됩니다.
저분자 이노베이터 CDMO 산업 개요
저분자 이노베이터 CDMO 시장은 세분화되고 있습니다. 이 시장의 지위를 강화하기 위해 각 회사는 업계의 존재를 확대하는 주목할만한 전략과 시책을 실시했습니다. 주요 시장 진출기업으로는 Eurofins Scientific, Cambrex Corporation, Catalent, Thermo Fisher Scientific, Jubilant Pharmova Limited 등이 있습니다.
기타 혜택
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간의 애널리스트 서포트
목차
제1장 서론
- 조사의 전제조건 및 시장의 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 저분자 의약품 수요 증가 및 파이프라인의 성장
- 만성 질환의 부담 증가
- 의약품 연구개발 투자 증가
- 시장 성장 억제요인
- 엄격한 정부 규제
- 아웃소싱의 컴플라이언스 문제
- Porter's Five Forces 분석
- 공급기업의 협상력
- 구매자 및 소비자의 협상력
- 신규 진입업자의 위협
- 대체품의 위협
- 경쟁 기업간 경쟁 관계의 강도
제5장 시장 세분화(시장 규모-달러)
- 제품별
- 저분자 원약
- 저분자 의약품
- 경구 고형 제제
- 반고형 제제
- 액제
- 기타
- 스테이지별
- 전임상
- 임상시험
- 제I상
- 제II상
- 제III상
- 제IV상
- 상업
- 최종 사용자별
- 제약 및 바이오테크놀러지
- 수탁연구기관
- 치료 영역별
- 심혈관 질환
- 종양
- 호흡기 질환
- 신경학
- 대사 질환
- 감염증
- 기타
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 영국
- 독일
- 프랑스
- 스페인
- 이탈리아
- 기타 유럽
- 아시아태평양
- 인도
- 일본
- 중국
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC 국가
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 프로파일
- Eurofins Scientific
- Cambrex Corporation
- Catalent
- Thermo Fisher Scientific Inc.
- Jubilant Pharmova Limited
- Lonza Group Ltd
- Wuxi AppTec
- Syngene International Limited
- Almac Group
- Piramal Pharma Solutions
- Recipharm AB
- Labcorp Drug Development
제7장 시장 기회 및 향후 동향
AJY 24.08.08The Small Molecules Innovator Contract Development And Manufacturing Organization Market size is estimated at USD 48.40 billion in 2024, and is expected to reach USD 69.90 billion by 2029, growing at a CAGR of 6.60% during the forecast period (2024-2029).
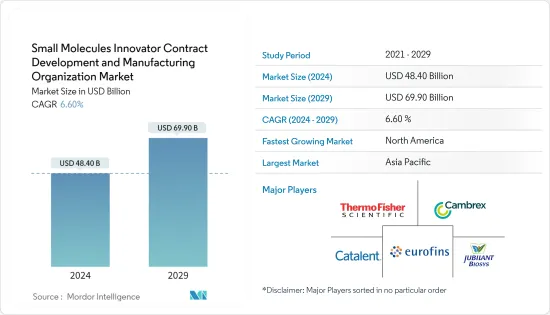
The market is driven by increasing demand for small molecule drugs, the growing pipeline of small molecule drugs, the increasing burden of chronic diseases, and rising pharmaceutical R&D investments. A robust pipeline of small molecule drugs means that pharmaceutical and biotech companies have more compounds in various stages of development. This creates a greater need for specialized services CDMOs provide, including formulation development, process optimization, and manufacturing. For instance, according to the Bio Industry Analysis Report published in February 2023, 47 small molecule new chemical entities (NCEs) were in the clinical pipeline as of 2022. Traditional systemic small-molecule antibiotics account for 97%, and topical small molecules account for 3% of the small-molecule pipeline NCEs.
Similarly, according to Clinicaltrials.gov, as of March 2024, there were 64 small molecules of drugs in phase II trials and 3 in the phase III trials. Hence, the growing pipeline often spans various therapeutic areas, addressing multiple medical conditions. This diversification in drug development increases the demand for CDMOs with expertise in different fields, further stimulating the market.
The substantial investment by pharmaceutical companies in research and development (R&D) of small molecule drugs has led to an increased demand for the services provided by contract development and manufacturing organizations (CDMOs). These organizations require specialized expertise and infrastructure to bring their drug candidates from the discovery phase through clinical trials. For instance, in September 2022, Cambrex completed the initial stage of its USD 30 million investment in its High Point, North Carolina facility for manufacturing small molecule active pharmaceutical ingredients (APIs). Furthermore, strategic collaboration for small molecule drug development is expected to drive market growth over the projected period. For instance, in January 2022, Sanofi and Exscientia entered a strategic research alliance and licensing pact for the purpose of developing up to 15 novel small molecule candidates across oncology and immunology, leveraging Exscientia's AI-driven platform utilizing actual patient samples.
Hence, the strong pipeline for small molecule drugs, increasing investment in developing small molecules, and key adoption of key strategies by market participants are expected to drive segment growth. However, stringent government regulations and compliance issues with outsourcing are anticipated to restrain the market growth over the projected period.
Small Molecules Innovator Contract Development And Manufacturing Organization Market Trends
The Neurology Segment is Expected to Hold Significant Share Between 2024 and 2029
The neurology segment in the small molecule innovators contract development and manufacturing organization (CDMO) market has been experiencing notable growth owing to growing awareness and understanding of neurological disorders, leading to an increased emphasis on the development of drugs targeting conditions like Alzheimer's, Parkinson's, epilepsy, and various other neurodegenerative diseases.
For instance, in July 2023, Biogen announced its small molecules pipeline drug Zuranolone (GABAA PAM) - Major depressive disorder (MDD) in phase III trials and BIIB131 (plasminogen activator) - Acute ischemic stroke in Phase II Trials. Similarly, UCB SA also reported that it has 4 small molecules of innovative drugs in the pipeline for neurology diseases, which include fenfluramine (5-HT agonist), doxecitine and doxribtimine (MT1621, nucleoside therapy), minzasolmin (a-syn-misfolding inhibitor), and STACCATO alprazolam (benzodiazepine) in various phase of development. Such a huge pipeline for small molecule innovator drugs is expected to create a huge demand for CDMO for process development, analytical testing, and manufacturing, which is expected to contribute to segment growth between 2024 and 2029.
An increase in CNS diseases, like Parkinson's disease, amyotrophic lateral sclerosis (ALS), Huntington chorea, and Alzheimer's disease, facilitates the demand for CRO services since the drug development of these diseases requires various clinical trial support, regulatory recommendations, etc. For instance, according to data published by Alzheimer's Disease Facts and Figures Annual Report 2023, over 6 million Americans suffered from Alzheimer's disease, a figure predicted to climb to almost 13 million within three decades.
Additionally, as per the above source, in 2023, the United States spent USD 345 billion in treating and managing Alzheimer's and other dementias. By 2050, these costs could rise to nearly USD 1 trillion. Thus, the high burden of the disease is creating demand for innovative and effective therapies, driving the growth of the studied market.
Furthermore, the data updated by the World Health Organization in March 2023 showed that globally, about 55 million people suffer from dementia; every year, there are nearly 10 million new cases of dementia filed. Additionally, Atlas of Multiple Sclerosis data updated in September 2023 showed that 2.9 million people live with multiple sclerosis in 2023 worldwide and 10 million with Parkinson's disease, as per the data published by the Parkinson's Foundation. These increasing burdens of CNS disorders are anticipated to drive the demand for central nervous system therapeutics and significant research activities between 2024 and 2029.
Pharmaceutical and biotechnology corporations are augmenting their focus on service expansion. For instance, in September 2023, Cellectricon expanded its neuroscience contract research portfolio with a new Neuroplasticity Services launched module set to accelerate drug discovery in therapeutic areas such as neuropsychiatry, epilepsy, and neurodegeneration.
Hence, increasing research and development activities, the presence of a strong pipeline, and strategic activities by the market players are expected to contribute to market growth between 2024 and 2029.
North America is Expected to Hold a Significant Market Share Between 2024 and 2029
North America, particularly the United States, is home to a well-established and robust pharmaceutical industry. This region has a high concentration of innovative pharmaceutical and biotech companies that are actively engaged in drug discovery and development. The high investment in R&D, the strong foothold of key market players, and rising grants from the National Institute of Health for developing novel therapeutics in the country also contribute to market growth.
The increasing investment by the pharmaceutical and biotechnology industry in the development of small-molecule drugs is expected to contribute to market growth. For instance, in May 2023, PharmEnable announced it had closed a Pre-Series A investment round of USD 7.5 million to develop the next generation of small molecule drugs against disease areas of high clinical need. Further, market players are engaging in service expansion, and collaboration is likely to propel the growth of the market. For instance, in April 2023, Phlow, a CDMO, announced that it had sealed USD 36 million through a Series B capital raise. Phlow stated that it would use the capital to expand its commercial offerings, which includes growing its CDMO program, called 'cdmoX. Additionally, in July 2022, Alliance Pharma, a company providing small and large-molecule bioanalytical services in the pharmaceutical and biopharmaceutical industry, closed the purchase of Drug Development Solutions (DDS) from LGC. Ampersand Capital Partners and KKR & Co. Inc.
Hence, increasing investment and strategic activities by the market players are expected to boost the market in the region between 2024 and 2029.
Small Molecules Innovator Contract Development And Manufacturing Organization Industry Overview
The small molecules innovator contract development and manufacturing organization market is fragmented in nature. In order to strengthen their position in the market, companies are implementing noteworthy strategies and measures to expand their industry presence. Some of the key market players are Eurofins Scientific, Cambrex Corporation, Catalent, Thermo Fisher Scientific Inc., and Jubilant Pharmova Limited.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definitions
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Demand for Small Molecule Drugs and Growing Pipeline of Small Molecule Drugs
- 4.2.2 Growing Burden of Chronic Diseases
- 4.2.3 Increasing Pharmaceutical R&D Investments
- 4.3 Market Restraints
- 4.3.1 Stringent Government Regulations
- 4.3.2 Compliance Issues with Outsourcing
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Bargaining Power of Suppliers
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Threat of New Entrants
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Product
- 5.1.1 Small Molecule API
- 5.1.2 Small Molecule Drug Product
- 5.1.2.1 Oral solid dose
- 5.1.2.2 Semi-Solid Dose
- 5.1.2.3 Liquid Dose
- 5.1.2.4 Others
- 5.2 By Stage
- 5.2.1 Preclinical
- 5.2.2 Clinical
- 5.2.2.1 Phase I
- 5.2.2.2 Phase II
- 5.2.2.3 Phase III
- 5.2.2.4 Phase IV
- 5.2.3 Commercial
- 5.3 By End User
- 5.3.1 Pharmaceutical and Biotechnology
- 5.3.2 Contract Research Organization
- 5.4 By Therapeutic Area
- 5.4.1 Cardiovascular disease
- 5.4.2 Oncology
- 5.4.3 Respiratory disorders
- 5.4.4 Neurology
- 5.4.5 Metabolic disorders
- 5.4.6 Infectious disease
- 5.4.7 Others
- 5.5 Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 United Kingdom
- 5.5.2.2 Germany
- 5.5.2.3 France
- 5.5.2.4 Spain
- 5.5.2.5 Italy
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 India
- 5.5.3.2 Japan
- 5.5.3.3 China
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of the Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Eurofins Scientific
- 6.1.2 Cambrex Corporation
- 6.1.3 Catalent
- 6.1.4 Thermo Fisher Scientific Inc.
- 6.1.5 Jubilant Pharmova Limited
- 6.1.6 Lonza Group Ltd
- 6.1.7 Wuxi AppTec
- 6.1.8 Syngene International Limited
- 6.1.9 Almac Group
- 6.1.10 Piramal Pharma Solutions
- 6.1.11 Recipharm AB
- 6.1.12 Labcorp Drug Development

















