
|
시장보고서
상품코드
1521871
아메리카의 암 면역치료 : 시장 점유율 분석, 산업 동향, 성장 예측(2024-2029년)America Cancer Immunotherapy - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
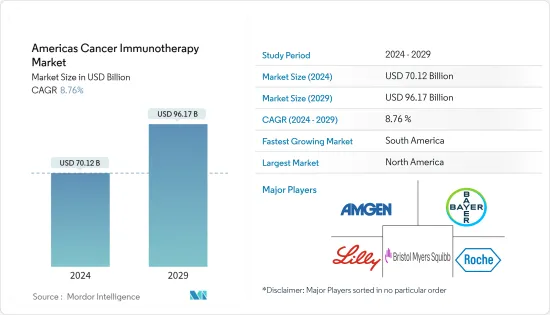
아메리카의 암 면역치료(America Cancer Immunotherapy) 시장 규모는 2024년에 701억 2,000만 달러로 추정되고, 2029년에는 961억 7,000만 달러에 이를 것으로 예측되며, 예측기간 중(2024-2029년) CAGR은 8.76%로 추이하며 성장할 것으로 예상됩니다.

아메리카의 암 면역치료 시장은 암 이환율의 상승, 연구개발 활동의 활성화, 새로운 치료법의 유효성과 정밀도의 향상에 의해 큰 성장 기회가 전망되고 있습니다. 또한 시장 진출기업의 자금 조달 활동 활성화와 여러 전략적 이니셔티브가 조사 기간 동안 시장 성장을 가속화할 것으로 예측됩니다.
아메리카 전체에서 암의 우려해야 할 부담 증가는 이 증상을 치료하기 위한 면역치료과 같은 혁신적인 암 치료에 대한 수요를 강화할 것으로 예상되고 있습니다. 예를 들어 미국암협회에 따르면 신규암 환자수는 2023년 190만명에서 2024년 200만명으로 급증할 것으로 추정되고 있습니다. 2023년에 진단된 암 중에서 가장 많았던 것은 유방암, 폐암, 혈액 악성 종양이었습니다.
마찬가지로 브라질 국립암 연구소의 추정에 따르면 2023-2025년 사이 비흑색종 피부암을 제외하고 약 70만 4,000명이 새롭게 암으로 진단될 것으로 예상되고 있습니다. 따라서 이 지역에서 이러한 우려해야 할 암의 부담은 효과적인 치료를 위한 면역치료과 같은 새로운 암 치료의 흡수를 가속시킬 것으로 예측됩니다.
이와 같이, 암 부담 증가는 면역치료과 같은 선진적인 암 치료 옵션의 채택을 가속시키고 있습니다. 게다가 암에 있어서의 면역치료의 몇개의 임상적 이점, 암 관리에 있어서의 면역치료의 역할 활용이, 조사 기간중 시장 성장을 한층 더 가속시킬 것으로 예상되고 있습니다.
예를 들어, International Journal of Molecular Sciences가 2023년 9월에 발표한 논문에 따르면, 면역치료은 최근 전립선암과 같은 치사적인 암에 대한 혁신적인 치료 옵션으로 등장했습니다. 면역치료은 표적 치료 및 개선된 임상 결과와 같은 몇몇 임상적 이점을 갖는다. 따라서 암 관리에 있어서의 면역치료의 역할을 활용함으로써 조사 기간 중 시장 확대가 가속될 것으로 예상됩니다.
게다가 정부의 지지적 법규제와 규제당국의 승인 확대는 조사기간 동안 산업 확대를 더욱 가속할 것으로 예측되고 있습니다. 예를 들어, 2024년 3월 미국 식품의약국(US FDA)은 Bristol-Myers Squib Eli Lilly and Company의 옵디보(니볼루맙)를 겜시타빈과 시스플라틴과 병용하여 성인의 전이성 요로 상피암의 첫 라인 치료로 승인했습니다. 이 승인은 전이성 요로 상피암 환자에 대해 승인된 중요한 면역치료과 화학요법의 조합의 하나가 되었습니다.
유사하게, 2022년 7월, 미국 FDA는 제넨텍의 생물학적 제제 승인 신청(BLA)을 수락하고 T 세포작용성 이중특이적 항체 mosunetuzumab의 우선 심사권을 부여했습니다. 이 면역 요법 약물은 성인 재발 또는 난치성 여포성 림프종(FL)을 적응증으로하는 첫 번째 클래스의 CD20xCD3 T 세포 작동 성 이중 특이성 항체입니다. 이러한 승인 취득 증가는 조사 기간 동안 시장 성장을 가속할 것으로 예상됩니다.
또한, 2024년 3월 Caring Cross와 Fundacao Oswaldo Cruz는 백혈병과 림프종을 포함한 다양한 유형의 암에 대한 줄기세포 유전자 치료와 CAR-T 세포 치료의 국내 생산을 개발하기 위해 협력했습니다.
아메리카의 암 면역치료 시장 동향
예측 기간 동안 유방암 부문이 상당한 시장 성장을 보일 것으로 예상
이 부문에는 유방암을 치료하는 암 면역 요법이 포함됩니다. 유방암은 여성에게 가장 많은 암 중 하나이며, 50세 이상의 여성이 이환될 가능성이 높습니다.
이 부문은 유방암의 이환율의 높이와 암 면역치료에 대한 수요 증가에 의해 예측기간 중에 성장할 것으로 예측되고 있습니다. 예를 들어 미국암협회에 따르면 유방암 부담이 크게 증가했고 총 환자수는 2023년 30만 0,590명에서 2024년 31만 3,510명으로 급증했습니다.
마찬가지로 남미 국가에서 유방암 부담 증가는 조사 기간 동안 부문 확대를 더욱 가속시킬 것으로 예상됩니다. 예를 들어 Global Cancer Observatory의 2023년 최신 데이터에 따르면 아르헨티나에서는 2022년에 약 2만 1,631명의 신규 유방암 사례가 진단되어 향후 수년간 증가할 것으로 예상되며 유방암의 부담은 아르헨티나의 다른 암 보다 훨씬 높습니다. 따라서 유방암에 대한 수요의 촉진이 조사 기간 동안 부문 성장을 가속할 것으로 예상됩니다.
이 부문의 성장을 견인하는 것은 유방암과 그 관리에 관한 의식 수준을 높이기 위한 공적기관이나 민간단체의 대처가 활발해지고 있는 것입니다. 예를 들어, 2023년 12월, Breast Cancer Canada는 유방암 치료의 최근 진보와 함께 유방암에 대한 인식을 촉진하기 위한 포털 사이트 Progress CONNECT를 개설했습니다.
또한 2022년 6월에는 Daiichi Sankyo Brazil이 브라질의 아마존 지역 여성을 대상으로 한 무료 유방암 검사를 실시했습니다. 이 노력은 여성 유방암에 대한 의식 수준을 강화하는 데 더욱 기여했습니다. 따라서 이러한 노력은 조사 기간 동안 부문 성장을 가속할 것으로 예상됩니다.
게다가 시장 진출 기업에 의한 최근의 동향은 보다 많은 유방암 면역치료의 가용성을 높일 것으로 예상되며, 조사 기간 중 부문 섭취를 촉진할 것으로 예측됩니다. 예를 들어 2024년 4월 미국 식품의약국(US FDA)은 AstraZeneca와 Daiichi Sankyo의 엔헤르투(트라스투주맙 델크스테칸)를 성인의 전이성 HER2 양성 유방암의 치료로 승인했습니다.
마찬가지로, 2022년 8월, Memorial Sloan Kettering Cancer Center는 최초의 표적 치료인 트라스투주맙 델크스테칸(T-DXd)의 승인을 미국 식품의약국에서 받았습니다. 이 암 면역 요법은 HER2 저가 유방암 환자의 관리를 목적으로합니다.
따라서 유방암의 우려할만한 증가, 유방암에 대한 의식의 고조, 시장 진출 기업에 의한 전략적 이니셔티브의 실시 등 위의 요인이 조사 대상 부문의 성장을 가속할 것으로 예상됩니다.
북미는 예측기간 중 상당한 성장이 예상
북미는 첨단 의료 인프라, 높은 암 이환율, 시장 진출기업의 전략적 이니셔티브에 의해 예측 기간 중에 큰 성장이 예상됩니다.
이 지역에서 암 환자 증가는 암 면역치료 수요를 높여 시장 성장을 가속할 것으로 예상됩니다. 예를 들어, 미국 암 협회가 2024년에 발표한 데이터에 따르면, 이 나라에서는 2023년에 5만 9,610명의 백혈병 환자가 보고되었지만, 2024년에는 약 6만 2,770명의 백혈병 환자가 보고 되었습니다. 이와 같이 이 지역에서 암의 우려해야 할 부담 증가는 조사기간 중 시장 확대를 촉진할 것으로 예측되고 있습니다.
북미는 견고한 의료 인프라와 지원적인 연구 환경을 가진 지역입니다. 최근 몇 년간 신규 암 치료 개발을 위한 연구 동향이 활발해지고 있으며, 이것이 조사 기간 중 시장 성장을 가속할 것으로 예측됩니다. 예를 들어, 2024년 3월에는 파듀 대학이 암 면역치료의 전신 전달을 강화하는 선진 나노입자를 개발했습니다.
또한 2024년 5월에는 워싱턴 대학 의료부의 과학자들이 백혈병 림프종 협회(LLS)로부터 500만 달러의 보조금을 받고 다양한 혈액암에 대한 신규 면역치료을 개발했습니다. 이러한 연구 활동은 조사 기간 동안 지역 시장의 성장을 가속시킬 것으로 예상됩니다.
또한, 이 나라 시장 진출 기업에 의한 최근의 동향은 이 지역 시장 성장을 뒷받침할 것으로 예상됩니다. 예를 들어, 2023년 3월, F. Hoffman La Roche Ltd(Roche Canada)는 재발 또는 난치성 확산성 대세포형 B 세포 림프종(DLBCL)의 관리를 목적으로 한 암 치료 콜롬비(주사용 그로피타마브)의 승인을 캐나다 보건부에서 받았습니다. 따라서 시장 진출기업에 의한 이러한 개척은 조사 기간 동안 시장 성장을 가속할 것으로 예상됩니다.
이와 같이 암에 대한 부담 증가, 자금 제공이나 조성금 증가, 시장 진출기업에 의한 전략적 이니셔티브 등, 상기와 같은 요인이 조사 기간 중에 동지역 시장 성장을 가속시킬 것으로 예측됩니다.
아메리카의 암 면역치료 산업 개요
아메리카의 암 면역치료 시장은 지역별로 여러 시장 진출 기업이 존재하기 때문에 그 성질상 단편적입니다. 각 회사는 R&D 및 제품 개발 투자 등 사업 기반을 강화하기 위한 전략적 노력을 하고 있습니다. 이 시장에 진입하는 주요 기업은 Amgen Inc., Bayer AG, Bristol-Myers Squib Eli Lilly and Company, F. Hoffman La Roche Ltd 등이 있습니다.
기타 혜택
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간의 애널리스트 서포트
목차
제1장 서론
- 조사 전제 조건 및 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 연구개발 활동의 활성화와 새로운 치료법의 유효성과 정밀도 향상
- 높은 암 이환율
- 시장 성장 억제요인
- 면역치료에 따른 부작용
- Porter's Five Forces 분석
- 신규 참가업체의 위협
- 구매자/소비자의 협상력
- 공급기업의 협상력
- 대체품의 위협
- 경쟁 기업간 경쟁 관계의 강도
제5장 시장 세분화(시장 규모-단위 : 달러)
- 치료 유형별
- 단클론항체
- 암 백신
- 면역조절제
- 면역 체크포인트 억제제
- 기타 치료 유형
- 용도별
- 전립선암
- 유방암
- 피부암
- 폐암
- 기타
- 최종 사용자별
- 병원 및 클리닉
- 암 연구센터
- 기타
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 프로파일
- Amgen Inc.
- Astellas Pharma Inc.
- AstraZeneca
- Bayer AG
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- F. Hoffman La Roche Ltd
- Merck and Co. Inc.
- Novartis AG
- Y-mAbs Therapeutics, Inc
- bluebird bio, Inc.
- Pfizer Inc.
- Gilead Sciences
- GSK plc
제7장 시장 기회 및 향후 동향
LYJThe Americas Cancer Immunotherapy Market size is estimated at USD 70.12 billion in 2024, and is expected to reach USD 96.17 billion by 2029, growing at a CAGR of 8.76% during the forecast period (2024-2029).
The American cancer immunotherapy market is anticipated to witness significant growth opportunities due to the rising burden of cancer, rising R&D activities, and the increasing effectiveness and accuracy of newer therapies. In addition, growing funding activities and several strategic initiatives undertaken by industry participants are projected to accelerate market growth over the study period.
The alarming increase in the burden of cancer across the Americas is expected to bolster the demand for innovative cancer therapies like immunotherapy to treat the condition. For instance, according to the American Cancer Society, the estimated number of new cancer cases ramped up from 1.9 million in 2023 to 2.0 million in 2024. The most common type of cancers diagnosed in 2023 were breast, lung, and hematological malignancies.
Similarly, according to the estimates from the National Cancer Institute of Brazil, around 704 thousand new cancer cases are expected to be diagnosed between 2023 and 2025, except non-melanoma skin cancer. Thus, such an alarming burden of cancer in the region is projected to accelerate the uptake of novel cancer therapies like immunotherapy for the effective treatment of disease.
Thus, the increased burden of cancers has accelerated the adoption of advanced cancer treatment options like immunotherapies. Moreover, several clinical benefits of immunotherapies in cancer and leveraging the role of immunotherapies in cancer management are further expected to accelerate market growth over the study period.
For instance, according to an article published by the International Journal of Molecular Sciences in September 2023, immunotherapy recently emerged as an innovative treatment option for lethal cancers like prostate cancer. It has several clinical benefits, including targeted therapy and improved clinical outcomes. Thus, leveraging the role of immunotherapy in the management of cancers is expected to accelerate market expansion over the study period.
Furthermore, supportive government legislation and growing regulatory approvals are further projected to accelerate industry expansion over the study period. For instance, in March 2024, the United States Food and Drugs Administration (U.S.FDA) approved Bristol Myers Squibb's Opdivo (nivolumab) in combination with gemcitabine and cisplatin for the first-line treatment of metastatic urothelial carcinoma in adults. This approval marked one of the key immunotherapy-chemotherapy combinations approved for patients with metastatic urothelial carcinoma.
Similarly, in July 2022, the US FDA accepted Genentech's Biologics License Application (BLA) and granted Priority Review for mosunetuzumab, a T-cell-engaging bispecific antibody. The immunotherapy drug is a first-in-class CD20xCD3 T-cell-engaging bispecific antibody intended for the management of relapsed or refractory (R/R) follicular lymphoma (FL) in adult patients. Thus, such growing regulatory approvals are projected to foster market growth over the study period.
Moreover, in March 2024, Caring Cross and Fundacao Oswaldo Cruz collaborated to develop domestic production of stem cell gene therapies and CAR-T cell therapies for various types of cancers, including leukemia, and lymphoma.
America Cancer Immunotherapy Market Trends
Breast Cancer Segment is Expected to Exhibit a Significant Market Growth Over the Forecast Period
The segment includes cancer immunotherapies for treating breast cancer, which is one of the most common cancers among women and most likely to affect women over the age of 50.
The segment is anticipated to grow during the forecast period owing to the high burden of breast cancer and increasing demand for cancer immunotherapies. For instance, according to the American Cancer Society, the burden of breast cancer increased significantly in the United States, and the total number of cases ramped from 300,590 in 2023 to 313,510 in 2024.
Similarly, the higher burden of breast cancer in South American countries is further expected to accelerate segment expansion over the study period. For instance, according to 2023 updated data from the Global Cancer Observatory, around 21,631 new breast cancer cases were diagnosed in Argentina in the year 2022, which is expected to increase over coming years, and the burden of breast cancer was much higher than other cancer types in Argentina. Thus, the fostering demand for breast cancer is expected to fuel segment growth over the study period.
The segment growth is expected to be driven by increased efforts from public and private organizations to foster awareness levels about breast cancer and its management. For instance, in December 2023, Breast Cancer Canada launched a portal, Progress CONNECT, to promote awareness about breast cancer along with recent advances in breast cancer treatment.
In addition, in June 2022, Daiichi Sankyo Brazil undertook a free breast cancer screening for women in Brazil's Amazon region. This initiative further helped to strengthen awareness levels of breast cancer among women. Thus, such initiatives are expected to foster segment growth over the study period.
Moreover, the recent developments by the market players are expected to boost the availability of more breast cancer immunotherapy, which is projected to facilitate segment uptake over the study period. For instance, in April 2024, the United States Food and Drug Administration (U.S.FDA) approved AstraZeneca and Daiichi Sankyo's Enhertu (trastuzumab deruxtecan) for the treatment of metastatic HER2-positive breast cancer in adults.
Similarly, in August 2022, Memorial Sloan Kettering Cancer Center received approval from the United States Food and Drug Administration for its first targeted therapy, Trastuzumab Deruxtecan (T-DXd). The cancer immunotherapy is intended for the management of HER2-low breast cancer patients.
Therefore, the above-mentioned factors, such as the alarming rise in breast cancer, increasing awareness for breast cancer, and several strategic initiatives undertaken by market players, are anticipated to drive the growth of the studied segment.
North America is Expected to Witness Significant Growth Over the Forecast Period
North America is expected to witness significant growth over the forecast period owing to the advanced medical infrastructure, the high burden of cancer, and the strategic initiatives the market players took.
The increase in cancer cases across the region is expected to elevate the demand for cancer immunotherapy, propelling market growth. For instance, according to the data published by the American Cancer Society in 2024, the country reported around 62,770 cases of leukemia in 2024 as compared to 59,610 in 2023. Thus, the alarming increase in the burden of cancer in the region is projected to facilitate market expansion over the study period.
North America is a region with a robust healthcare infrastructure and a supportive research environment. In recent years, the research activities for novel cancer treatment development have increased, and this is projected to accelerate market growth over the study period. For instance, in March 2024, Purdue University, United States, developed advanced nanoparticles to enhance the systemic delivery of cancer immunotherapy.
In addition, in May 2024, the scientists of Washington University School of Medicine received a grant of USD 5 million from the Leukemia & Lymphoma Society (LLS) to develop novel immunotherapies for various blood cancers. Such research activities are expected to accelerate regional market growth over the study period.
Moreover, the recent developments by the market players in the country are expected to boost the market growth in the region. For instance, in March 2023, Hoffmann-La Roche Limited (Roche Canada) received authorization from Health Canada for its cancer therapy, COLUMVI (glofitamab for injection), intended for the management of relapsed or refractory diffuse large B-cell lymphoma (DLBCL). Thus, such developments undertaken by industry participants are expected to foster market growth over the study period.
Thus, the above-mentioned factors, such as the increased burden of cancer, growing funding/grants, and several strategic initiatives undertaken by industry participants, are projected to accelerate market growth in the region over the study period.
America Cancer Immunotherapy Industry Overview
America's cancer immunotherapy market is fragmented in nature due to the presence of several market players operating regionally. Companies are undertaking several strategic initiatives to strengthen their business avenues, such as investing in research and product development. Some of the leading players operating in the market include Amgen Inc., Bayer AG, Bristol-Myers Squib Eli Lilly and Company, and F. Hoffman La Roche Ltd.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising R&D Activities and Increasing Effectivity and Accuracy Of Newer Therapies
- 4.2.2 High Burden of Cancer
- 4.3 Market Restraints
- 4.3.1 Side Effects Associated With the Immunotherapy
- 4.4 Porter's Five Force Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market size by Value in USD)
- 5.1 By Therapy Type
- 5.1.1 Monoclonal Antibodies
- 5.1.2 Cancer Vaccines
- 5.1.3 Immunomodulators
- 5.1.4 Immune Check Point Inhibitors
- 5.1.5 Other Therapy Types
- 5.2 By Application
- 5.2.1 Prostate Cancer
- 5.2.2 Breast Cancer
- 5.2.3 Skin Cancer
- 5.2.4 Lung Cancer
- 5.2.5 Other Applications
- 5.3 By End Users
- 5.3.1 Hospitals and Clinics
- 5.3.2 Cancer Research Centers
- 5.3.3 Other End Users
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 South America
- 5.4.2.1 Brazil
- 5.4.2.2 Argentina
- 5.4.2.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Amgen Inc.
- 6.1.2 Astellas Pharma Inc.
- 6.1.3 AstraZeneca
- 6.1.4 Bayer AG
- 6.1.5 Bristol-Myers Squibb Company
- 6.1.6 Eli Lilly and Company
- 6.1.7 F. Hoffman La Roche Ltd
- 6.1.8 Merck and Co. Inc.
- 6.1.9 Novartis AG
- 6.1.10 Y-mAbs Therapeutics, Inc
- 6.1.11 bluebird bio, Inc.
- 6.1.12 Pfizer Inc.
- 6.1.13 Gilead Sciences
- 6.1.14 GSK plc

















