
|
시장보고서
상품코드
1883721
세포치료용 배지 시장 : 업계 동향과 세계 예측(-2035년) - 제품 유형별, 세포치료 유형별, 사업 규모별, 최종사용자 유형별, 지역별Cell Therapy Media Market: Industry Trends and Global Forecasts, Till 2035 - Distribution by Type of Product, Type of Cell Therapy, Scale of Operation, Type of End User and Key Geographical Regions |
||||||
세포치료용 배지 시장 : 개요
Roots Analysis의 조사에 따르면 세계의 세포치료용 배지 시장은 현재 16억 달러에서 2035년까지 45억 달러로 성장하며, 예측 기간(-2035년)의 CAGR은 11.1%로 추정되고 있습니다.
시장 규모 및 기회 분석은 다음 매개 변수를 기반으로 세분화됩니다.
제품 유형
- 배양배지, 키트
- 세포배양 시약
- 세포외 매트릭스
세포치료 유형
- T 세포 치료
- 줄기세포치료
- 수지상세포치료
- NK세포치료
사업 규모
- 임상
- 상업
최종사용자 유형
- 산업
- 비산업
주요 지역
- 북미
- 유럽
- 아시아태평양
- 중동 및 북아프리카
- 라틴아메리카
세포치료용 배지 시장 : 성장과 동향
암, 희귀질환, 만성질환 치료에서 FDA 승인을 받은 세포치료제의 놀라운 발전과 입증된 효과로 인해 세포치료제 시장은 의료 분야 이해관계자들로부터 큰 관심을 받고 있습니다. 흥미롭게도 2019년 이후 주로 세포치료에 초점을 맞춘 1,000개 이상의 임상시험이 등록되었습니다. 또한 전 세계 각 지역에서 35개 이상의 세포치료제 및 유전자치료제가 시장에 출시되고 있습니다. 최근 세포치료제의 승인 사례로는 Breyanzi(R), Carvykti(TM), Abecma(R) 등이 있습니다.
세포치료용 소모품 제조 관련 규제가 강화됨에 따라 이 분야 개발자의 90% 이상이 고품질의 원료를 제공할 수 있는 전문성을 갖춘 공급업체로부터 배양배지, 키트, 시약, 세포외 매트릭스를 외부에서 조달하는 것을 선택하고 있습니다. 현재 80개 이상의 기업이 450개 이상의 연구용 및/또는 치료용 원료를 제공합니다. 또한 특정 기업은 T세포, 줄기세포, 수지상세포, NK세포 등 다양한 인체 세포용 소모품을 제조할 수 있는 GMP 인증 시설을 보유하고 있다고 주장하고 있습니다.
세포치료의 발전과 함께 업계내 혁신과 파트너십이 증가하면서 환자 치료에 혁명을 일으키고 재생의료의 범위를 확장할 수 있는 새로운 치료 옵션을 창출할 것으로 기대됩니다.
세포치료용 배지 시장 : 주요 연구 결과
이 보고서는 세포치료용 배지 시장의 현황을 상세하게 분석하고 업계내 잠재적인 성장 기회를 파악합니다. 주요 조사 결과는 다음과 같습니다.
전문성을 바탕으로 연구 및 치료 목적으로 450개 이상의 키트, 배지, 시약, 세포외 매트릭스 등 다양한 소모품 공급업체가 생산하고 있습니다.
2. 전 세계 80개 이상의 기업이 시장에 진출해 있으며, 이들 이해관계자의 대부분은 북미에 기반을 둔 신생 기업입니다.
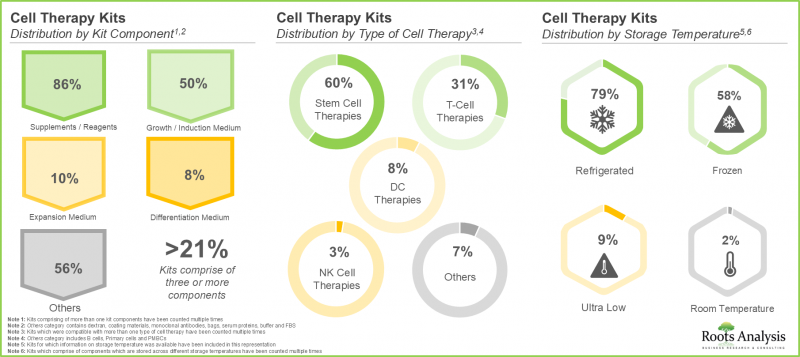
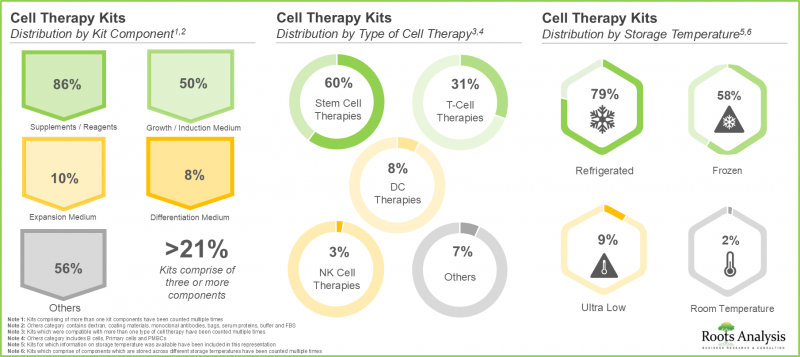
3. 세포치료 분야에서 사용 가능한 키트의 대부분은 각종 시약으로 구성되어 있으며, 그 중 60%는 줄기세포 치료용, 약 80%는 냉장보관이 요구됩니다.
4. 배지 개발 기업은 다양한 세포치료용 제품을 제공하고 있으며, 이들 기업의 약 90%가 100-500ml 용량 범위의 배지를 공급하고 있습니다.
5. 주로 세포치료용 시약은 세포의 증식 및 증폭을 포함한 광범위한 기능에서 발견 단계의 조작 규모에서 사용하기 위한 것입니다.
6. 일반적으로 1-1.5년의 유통기한을 가진 매트릭스 시장 상황은 ECM 코팅의 유형과 제형 유형에 따라 고르게 분포되어 있습니다.
7. 경쟁 우위를 확보하기 위해 세포치료용 소모품 공급업체들은 기존 기술을 고도화하고 제품 포트폴리오를 확장하고 있습니다.
8. 많은 기업이 기존 역량을 강화하기 위해 제휴, 인수, 사업 확장 등의 전략적 노력을 기울이고 있습니다.
9. 업계 이해관계자들은 각 사의 제품 라인업을 더욱 확대하기 위해 이 분야에 종사하는 틈새/전문 기업과의 전략적 제휴를 지속적으로 구축할 것으로 예측됩니다.
10. 수년 동안 업계 이해관계자들은 세포치료용 원료의 개발을 더욱 촉진하기 위해 다양한 노력을 통해 확고한 브랜드 입지를 구축해 왔습니다.
11. 세포치료제 제조 공정에서 소모품의 채택에 있으며, 비용은 결정적인 요소입니다.
12. 2035년에는 상업적 규모의 운영이 세포치료용 소모품 총 수요의 75%를 차지할 것으로 예측됩니다. 이는 여러 세포치료제 승인이 급증할 것으로 예상되기 때문입니다.
13. 동물 유래 성분을 함유한 제제에서 동물 유래 성분을 함유하지 않은 제제로의 패러다임 전환과 엄격한 규제 가이드라인이 맞물려 세포치료용 소모품 시장은 연평균 11.1%의 성장률로 확대될 것으로 예측됩니다.
세포치료용 배지 시장 : 주요 부문
세포 치료용 배지 시장에서 가장 빠르게 성장하는 분야는 세포 외 매트릭스입니다.
제품 유형에 따라 시장은 배양 배지, 키트, 세포배양 시약, 세포 외 매트릭스로 분류됩니다. 현재 세포치료용 배지 시장의 대부분을 배양배지가 차지하고 있다는 점은 주목할 만합니다.
예측 기간 중 T세포 치료제가 세포치료용 배지 시장을 독점할 것으로 예측됩니다.
세포치료의 유형에 따라 시장은 T세포치료, 줄기세포치료, 수지상세포치료, NK세포치료로 분류됩니다. 특히 주목할 만한 점은 예측 기간 중 NK 세포치료용 세포치료 소모품 시장이 비교적 높은 CAGR로 성장할 것으로 예상된다는 점입니다. 이는 현재 다양한 질환 적응증에 대해 75개 이상의 NK세포치료제 관련 임상연구가 진행 중이기 때문입니다. 향후 수년간 이 숫자는 더욱 증가할 것으로 예상되며, 이러한 치료법 시장 기회를 촉진할 가능성이 높습니다.
사업 규모별로는 예측 기간 중 상업적 규모가 세포치료용 배지 시장을 주도할 것으로 예측됩니다.
사업 규모에 따라 시장은 임상 규모와 상업 규모로 세분화됩니다. 주목할 만한 점은 상업적 규모의 세포치료용 배지 시장이 가까운 미래에 시장을 주도할 가능성이 높다는 점입니다.
세포 치료용 배지 시장에서 가장 큰 점유율을 차지하고 있는 산업 진출기업 부문
최종사용자 유형에 따라 시장은 산업 및 비산업용으로 시장 세분화됩니다. 특히 주목할 만한 점은 산업계 진입기업을 위한 세포치료용 배지 시장이 가까운 미래에 시장을 주도할 가능성이 높다는 점입니다. 이는 승인된 치료법의 대부분이 이러한 진입기업에 의해 개발되었으므로 산업계 진입기업이 전체 상업적 시장에 큰 기여를 하고 있기 때문입니다.
북미가 시장에서 가장 큰 점유율을 차지합니다.
주요 지역별로 북미, 유럽, 아시아태평양, 중동 및 북아프리카, 라틴아메리카로 구분됩니다. 특히 주목할 만한 점은 라틴아메리카 시장이 향후 수년간 더 높은 CAGR로 성장할 것으로 예상된다는 점입니다.
세포치료용 배지 시장의 대표 기업 사례
- BD Biosciences
- Bio-Techne
- CellGenix
- Corning
- 어바인 사이언티픽(FUJIFILM 인수)
- Lonza
- Miltenyi Biotech
- Sartorius
- STEMCELL Technologies
- Thermo Fisher Scientific
1차 조사 개요
본 조사에서 제시된 견해와 인사이트는 여러 이해관계자와의 논의를 바탕으로 한 것입니다. 본 조사 보고서에는 다음과 같은 업계 관계자와의 상세한 인터뷰 기록이 수록되어 있습니다.
- A사 사업개발 담당 부사장
- B사 최고운영책임자(COO)
- C사 세포배양-면역학 부문 연구개발본부장
- D사 동물세포배양 부문 연구개발 보조 관리자
목차
제1장 서문
제2장 개요
제3장 서론
- 문맥과 배경
- 세포치료 입문
- 세포치료와 기타 바이오의약품의 비교
- 세포치료 제품의 분류
- 세포치료의 개발과 제조의 개요
- 세포치료의 개발과 제조에서 원재료의 역할
- 세포치료 소모품의 유형
- 세포치료 소모품의 제조에 관련된 주요 과제
- 향후 전망
제4장 시장 개요
- 챕터 개요
- 세포치료 키트 프로바이더 리스트
- 세포치료용 배지 프로바이더 리스트
- 세포치료 시약 프로바이더 리스트
- 세포치료 세포외 매트릭스 프로바이더 리스트
제5장 기업 경쟁력 분석
- 챕터 개요
- 주요 전제와 파라미터
- 조사 방법
- 세포치료 소모품 프로바이더 : 기업 경쟁력 분석
- 세포치료 키트 프로바이더
- 세포치료용 배지 프로바이더
- 북미에 기반을 둔 세포치료용 배지 프로바이더
- 유럽에 기반을 둔 세포치료용 배지 프로바이더
- 아시아태평양에 기반을 둔 세포치료 미디어 프로바이더
- 세포치료 시약 프로바이더
- 세포치료 세포외 매트릭스 프로바이더
제6장 주요 업계 참여 기업의 브랜드 포지셔닝
제7장 기업 개요
- 챕터 개요
- STEMCELL Technologies
- Miltenyi Biotec
- Thermo Fisher Scientific
- Bio-Techne
- Irvine Scientific
- Lonza
- Sartorius
- BD Biosciences
- Corning
- CellGenix
제8장 최근 동향과 구상
- 챕터 개요
- 파트너십 모델
- 세포치료용 소모품 : 파트너십과 협업
- 세포치료용 소모품 : 합병과 인수
- 세포치료용 소모품 : 최근 확대
제9장 세포치료 소모품 프로바이더를 위한 적절한 파트너 분석
- 챕터 개요
- 채점 기준과 주요 전제
- 범위와 조사 방법
- 세포치료 소모품 프로바이더에서의 주요 잠재적 전략적 파트너
제10장 ROOTS ANALYSIS 가격 전략
- 챕터 개요
- ROOTS ANALYSIS 가격 전략적 프레임워크
제11장 수요 분석
제12장 시장 예측과 기회 분석
- 챕터 개요
- 주요 전제와 조사 방법
- 2035년까지의 세계 세포치료 소모품 시장
- 세포치료용 소모품 시장 : 제품 유형별 분석
- 세포치료용 소모품 시장 : 세포치료 유형별 분석
- 세포치료용 소모품 시장 : 사업 규모별 분석
- 세포치료용 소모품 시장 : 최종사용자 유형별 분석
- 세포치료용 소모품 시장 : 지역별 분석
제13장 향후 동향과 향후 성장 기회
- 챕터 개요
- 세포배양 배지에 관한 새로운 동향
- 세포치료 제조 프로세스의 자동화
- 세포치료 제조에서 일회용 시스템과 기술
제14장 결론
제15장 인터뷰 기록
제16장 부록 I : 표 데이터
제17장 부록 II : 기업 및 조직 리스트
KSA 25.12.17CELL THERAPY MEDIA MARKET: OVERVIEW
As per Roots Analysis, the global cell therapy media market is estimated to grow from USD 1.6 billion in the current year to USD 4.5 billion by 2035, at a CAGR of 11.1% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Product
- Culture Media, Kits
- Cell Culture Reagents
- Extracellular Matrices
Type of Cell Therapy
- T-Cell Therapy
- Stem Cell Therapy
- Dendritic Cell Therapy
- NK Cell Therapy
Scale of Operation
- Clinical
- Commercial
Type of End User
- Industry
- Non-Industry
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and North Africa
- Latin America
CELL THERAPY MEDIA MARKET: GROWTH AND TRENDS
Fueled by significant advancements and the established efficacy of FDA-approved cell therapies for treating cancer, rare diseases, and chronic conditions, the cell therapy media market has garnered significant interest from stakeholders in the healthcare sector. Interestingly, over 1,000 clinical trials primarily focused on cell therapies have been registered since 2019. Further, more than 35 cell and gene therapies have been brought to market in different regions around the world. Recent cell therapy approval includes Breyanzi(R), Carvykti(TM), and Abecma(R).
With rising regulatory stringency associated with producing consumables for cell therapy, more than 90% of developers in this field opt to outsource culture media, kits, reagents, and extracellular matrices to suppliers with the required expertise to provide high-quality raw materials. Currently, over 80 companies are providing more than 450 research and / or therapeutic grade raw materials. Moreover, certain companies claim to have GMP-certified facilities for manufacturing consumables intended for various human cells, such as T-cells, stem cells, dendritic cells, and NK cells.
With the ongoing evolution of cell therapy, we expect a rise in innovation and partnerships in the industry, leading to new therapeutic alternatives with the potential to revolutionize patient care and broaden the scope of regenerative medicine.
CELL THERAPY MEDIA MARKET: KEY INSIGHTS
The report delves into the current state of the cell therapy media market and identifies potential growth opportunities within the industry. Some key findings from the report include:
1. Leveraging their expertise, over 450 types of kits, media, reagents and extracellular matrices have been manufactured by consumable providers for research and therapeutic purposes.
2. The market features the presence of over 80 firms across the globe; the majority of these stakeholders are emerging players based in North America.
3. A larger proportion of the kits available in the cell therapy domain comprises different types of reagents; of these, 60% are intended for use with stem cell therapies and nearly 80% are stored in refrigerated conditions.
4. Media developers are offering products for a broad range of cell therapies; nearly 90% of such players are providing media in the volume range of 100 to 500 ml.
5. Predominantly, the cell therapy reagents are intended to be used at the discovery scale of operation for a wide spectrum of functions, including cell expansion and proliferation.
6. The market landscape of matrices, which typically have a shelf life of 1 to 1.5 years, is well distributed in terms of type of ECM coating and type of formulation.
7. In pursuit of gaining a competitive edge, cell therapy consumable providers are upgrading their existing technologies and expanding their product portfolios.
8. Many companies have undertaken strategic initiatives, including partnerships, acquisitions and expansions, to augment their existing capabilities.
9. We expect industry stakeholders to continue to forge strategic alliances with niche / specialized players engaged in this domain to further augment their respective product offerings.
10. Over the years, stakeholders within this industry have established strong brand positions by undertaking a range of initiatives to further advance the development of raw materials for cell therapies.
11. Cost is a key determinant for the adoption of consumables in a cell therapy manufacturing process.
12. In 2035, the commercial scale of operation is likely to account for 75% of the total demand for cell therapy consumables; this is attributed to the expected surge in the anticipated approvals of multiple cell therapies.
13. A paradigm shift from animal-based to animal component free formulations, combined with stringent regulatory guidelines, is likely to drive the growth of the cell therapy consumables market at an annualized rate of 11.1%.
CELL THERAPY MEDIA MARKET: KEY SEGMENTS
Extracellular Matrices is the Fastest Growing Segment in the Cell Therapy Media Market
Based on the type of product, the market is segmented into culture media, kits, cell culture reagents and extracellular matrices. It is worth highlighting that majority of the current cell therapy media market is captured by culture media.
T-Cell Therapy is Likely to Dominate the Cell Therapy Media Market During the Forecast Period
Based on the type of cell therapy, the market is segmented into T-Cell therapy, stem cell therapy, dendritic cell therapy and NK cell therapy. It is worth highlighting that the cell therapy consumables market for NK cell therapies is likely to grow at a relatively higher CAGR, during the forecast period. This can be attributed to the fact that currently, more than 75 NK cell therapy focused clinical studies are being evaluated for a myriad of disease indications. In the coming years, this number is anticipated to increase further, and this is likely to boost the market opportunity for such therapies.
By Scale of Operation, Commercial Scale is Likely to Dominate the Cell Therapy Media Market During the Forecast Period
Based on the scale of operation, the market is segmented into clinical and commercial scales. It is worth highlighting that the commercial scale cell therapy media market is likely to drive the market in the near future.
Industry Players Segment Accounts for the Largest Share of the Cell Therapy Media Market
Based on the type of end user, the market is segmented into industry and non-industry. It is worth highlighting that the cell therapy media market for industry players is likely to drive the market in the near future. This can be attributed to the fact that the industry players contribute significantly to the overall commercial market as majority of the approved therapies have been developed by such players.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific, Middle East and North Africa, and Latin America. It is worth highlighting that, over the years, the market in Latin America is expected to grow at a higher CAGR.
Example Players in the Cell Therapy Media Market
- BD Biosciences
- Bio-Techne
- CellGenix
- Corning
- Irvine Scientific (Acquired by FUJIFILM)
- Lonza
- Miltenyi Biotech
- Sartorius
- STEMCELL Technologies
- Thermo Fisher Scientific
Primary Research Overview
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:
- Vice President of Business Development, Company A
- Chief Operating Officer, Company B
- Director R&D, Cell Culture and Immunology, Company C
- Assistant R&D Manager, Animal Cell Culture, Company D
CELL THERAPY MEDIA MARKET: RESEARCH COVERAGE
- Market Forecast and Opportunity Analysis: The report features an in-depth analysis of the cell therapy media market, focusing on key market segments, including [A] type of product, [B] type of cell therapy, [C] scale of operation, [D] type of end user and [E] key geographical regions.
- Market Landscape: A comprehensive evaluation of companies offering cell culture consumables and cell culture media, considering various parameters, [A] such as year of establishment, [B] company size (in terms of number of employees), [C] location of headquarters, [D] type of product, [E] number and location of consumable facilities, [F] accreditations received, [G] type of end-user, [H] cell culture media compatibility, [I] type of cell therapy, [J] type of function, [K] kit components, [L] type of ECM coating, [M] type of formulation, [N] shelf life, [O] scale of operation, [P] application area, and [Q] storage temperature.
- Company Competitiveness Analysis: A comprehensive competitive analysis of companies operating in the cell culture consumable and cell culture media market, examining factors such as [A] supplier strength, [B] portfolio strength and [C] number of products offered.
- Brand Positioning Analysis: A detailed brand positioning analysis of prominent industry players, highlighting the current perceptions regarding their proprietary brands across different consumable classes.
- Company Profiles: In-depth profiles of key industry players in cell therapy consumables and cell culture media market, focusing on [A] company overviews, [B] product portfolio, [C] consumable facilities, [D] recent developments and an [E] informed future outlook.
- Recent Developments and Initiatives: An analysis of recent developments within the cell culture consumables and cell culture media market, [A] covering partnerships and collaborations, [B] mergers and acquisitions, and [B] expansion initiatives.
- Likely Partner Analysis: A detailed evaluation of over 250 cell therapy developers that are most likely to collaborate with cell culture consumables and cell culture media providers. This analysis considers various relevant parameters, including [A] developer strength (which takes into account a company's size and its experience in this field), [B] pipeline strength and [C] maturity (based on the number of pipeline drugs and affiliated stage of development), and availability of other cell therapy capabilities.
- Roots Analysis Pricing Strategy: A proprietary Roots Analysis competitive pricing framework, which analyzes the competitive position of various companies engaged in cell culture consumables and cell culture media market, by taking into consideration the prices and features of their consumable offerings (such as media and extracellular matrices).
- Demand Analysis: Informed estimates of the annual demand for cell culture consumables and cell culture media (in terms of volume of media required for total number of cells), based on [A] scale of operation and [B] key geographical regions.
- Upcoming Trends And Future Growth Opportunities: A comprehensive analysis of emerging trends and future growth prospects in the cell culture consumables and media market. It includes details related to the significance of automation in cell therapy manufacturing processes and the benefits of single use technologies for the production of cell therapies.
KEY QUESTIONS ANSWERED IN THIS REPORT
- What are the factors driving the cell therapy consumables market?
- How many players are engaged in offering cell culture media for manufacturing cell therapies?
- How many players are engaged in offering kits for manufacturing cell therapies?
- How many media products are available in the market for culturing cell therapies?
- What are the partnership and collaboration trends observed in the cell therapy consumables domain?
- Which geographical segment captures the largest market share in the current cell therapy consumables market?
- Which type of product contributes to the largest share of the cell therapy consumables market?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Key Market Insights
- 1.3. Scope of the Report
- 1.4. Research Methodology
- 1.5. Frequently Asked Questions
- 1.6. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Context and Background
- 3.2. Introduction to Cell Therapies
- 3.3. Comparison of Cell Therapies with Other Biopharmaceuticals
- 3.4. Classification of Cell Therapy Products
- 3.5. Overview of Cell Therapy Development and Manufacturing
- 3.6. Role of Raw Materials in Cell Therapy Development and Manufacturing
- 3.7. Types of Cell Therapy Consumables
- 3.8. Key Challenges Associated with Manufacturing of Cell Therapy Consumables
- 3.9. Future Perspectives
4. MARKET OVERVIEW
- 4.1. Chapter Overview
- 4.2. List of Cell Therapy Kit Providers
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Location of Headquarters
- 4.2.4. Analysis by Location of Kits Manufacturing Facilities
- 4.2.5. Analysis by Certifications / Accreditations Received
- 4.2.6. Analysis by Type of End-User
- 4.2.7. Analysis by Type of Cell Therapy
- 4.2.8. Analysis by Type of Function
- 4.2.9. Analysis by Kit Components
- 4.2.10. Analysis by Storage Temperature
- 4.2.11. Analysis by Scale of Operation
- 4.2.12. Analysis by Application Area
- 4.2.13. Analysis by Application Area and Geography
- 4.3. List of Cell Therapy Media Providers
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Location of Headquarters
- 4.3.4. Analysis by Location of Media Manufacturing Facilities
- 4.3.5. Analysis by Certifications / Accreditations Received
- 4.3.6. Analysis by Type of End-User
- 4.3.7. Analysis by Type of Cell Therapy
- 4.3.8. Analysis by Media Compatibility
- 4.3.9. Analysis by Type of Function
- 4.3.10. Analysis by Storage Temperature
- 4.3.11. Analysis by Volume of Media
- 4.3.12. Analysis by Scale of Operation
- 4.3.13. Analysis by Application Area
- 4.3.14. Analysis by Application Area and Geography
- 4.4. List of Cell Therapy Reagent Providers
- 4.4.1. Analysis by Year of Establishment
- 4.4.2. Analysis by Company Size
- 4.4.3. Analysis by Location of Headquarters
- 4.4.4. Analysis by Location of Reagent Manufacturing Facilities
- 4.4.5. Analysis by Certifications / Accreditations Received
- 4.4.6. Analysis by Type of End-User
- 4.4.7. Analysis by Type of Cell Therapy
- 4.4.8. Analysis by Type of Function
- 4.4.9. Analysis by Storage Temperature
- 4.4.10. Analysis by Volume of Reagent
- 4.4.11. Analysis by Scale of Operation
- 4.4.12. Analysis by Application Area
- 4.4.13. Analysis by Application Area and Geography
- 4.5. List of Cell Therapy Extracellular Matrix Providers
- 4.5.1. Analysis by Year of Establishment
- 4.5.2. Analysis by Company Size
- 4.5.3. Analysis by Location of Headquarters
- 4.5.4. Analysis by Location of Extracellular Matrix Manufacturing Facilities
- 4.5.5. Analysis by Certifications / Accreditations Received
- 4.5.6. Analysis by Type of End-User
- 4.5.7. Analysis by Type of Stem Cell Therapy
- 4.5.8. Analysis by Type of Function
- 4.5.9. Analysis by Type of ECM Coating
- 4.5.10. Analysis by Type of Formulation
- 4.5.11. Analysis by Shelf Life
- 4.5.12. Analysis by Storage Temperature
- 4.5.13. Analysis by Volume of Extracellular Matrix
- 4.5.14. Analysis by Scale of Operation
- 4.5.15. Analysis by Application Area
- 4.5.16. Analysis by Application Area and Geography
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Key Assumptions and Parameters
- 5.3. Methodology
- 5.4. Cell Therapy Consumables Providers: Company Competitiveness Analysis
- 5.5. Cell Therapy Kit Providers
- 5.6. Cell Therapy Media Providers
- 5.6.1. Cell Therapy Media Providers based in North America
- 5.6.2. Cell Therapy Media Providers based in Europe
- 5.6.3. Cell Therapy Media Providers based in Asia-Pacific
- 5.7. Cell Therapy Reagent Providers
- 5.8. Cell Therapy Extracellular Matrix Providers
6. BRAND POSITIONING OF KEY INDUSTRY PLAYERS
- 6.1. Chapter Overview
- 6.2. Scope and Methodology
- 6.3. Brand Positioning: STEMCELL Technologies
- 6.4. Brand Positioning: Miltenyi Biotec
- 6.5. Brand Positioning: Thermo Fisher Scientific
- 6.6. Brand Positioning: Takara Bio
- 6.7. Brand Positioning: GeminiBio
7. COMPANY PROFILES
- 7.1. Chapter Overview
- 7.2. STEMCELL Technologies
- 7.2.1. Company Overview
- 7.2.2. Product Portfolio
- 7.2.3. Recent Developments and Future Outlook
- 7.3. Miltenyi Biotec
- 7.3.1. Company Overview
- 7.3.2. Product Portfolio
- 7.3.3. Recent Developments and Future Outlook
- 7.4. Thermo Fisher Scientific
- 7.4.1. Company Overview
- 7.4.2. Product Portfolio
- 7.4.3. Recent Developments and Future Outlook
- 7.5. Bio-Techne
- 7.5.1. Company Overview
- 7.5.2. Product Portfolio
- 7.5.3. Recent Developments and Future Outlook
- 7.6. Irvine Scientific
- 7.6.1. Company Overview
- 7.6.2. Product Portfolio
- 7.6.3. Recent Developments and Future Outlook
- 7.7. Lonza
- 7.7.1. Company Overview
- 7.7.2. Product Portfolio
- 7.7.3. Recent Developments and Future Outlook
- 7.8. Sartorius
- 7.8.1. Company Overview
- 7.8.2. Product Portfolio
- 7.8.3. Recent Developments and Future Outlook
- 7.9. BD Biosciences
- 7.9.1. Company Overview
- 7.9.2. Product Portfolio
- 7.9.3. Recent Developments and Future Outlook
- 7.10. Corning
- 7.10.1. Company Overview
- 7.10.2. Product Portfolio
- 7.10.3. Recent Developments and Future Outlook
- 7.11. CellGenix
- 7.11.1. Company Overview
- 7.11.2. Product Portfolio
- 7.11.3. Recent Developments and Future Outlook
8. RECENT DEVELOPMENTS AND INITIATIVES
- 8.1. Chapter Overview
- 8.2. Partnership Models
- 8.3. Cell Therapy Consumables: Partnerships and Collaborations
- 8.3.1. Analysis by Year of Partnership
- 8.3.2. Analysis by Type of Partnership
- 8.3.3. Analysis by Year and Type of Partnership
- 8.3.4. Analysis by Type of Product
- 8.3.5. Analysis by Type of Partnership and Type of Product
- 8.3.6. Analysis by Type of Cell Therapy
- 8.3.7. Analysis by Type of Product and Type of Cell Therapy
- 8.3.8. Most Active Players: Analysis by Number of Partnerships
- 8.3.9. Analysis by Region
- 8.3.9.1. Intercontinental and Intracontinental Agreements
- 8.3.9.2. Local and International Agreements
- 8.4. Cell Therapy Consumables: Mergers and Acquisitions
- 8.4.1. Cumulative Year-wise Trend of Mergers and Acquisitions
- 8.4.2. Analysis by Type of Agreement
- 8.4.3. Analysis by Key Value Drivers
- 8.4.4. Analysis by Year of Acquisition and Key Value Drivers
- 8.5. Cell Therapy Consumables: Recent Expansions
- 8.5.1. Analysis by Year of Expansion
- 8.5.2. Analysis by Type of Expansion
- 8.5.3. Analysis by Year and Type of Expansion
- 8.5.4. Analysis by Type of Product
- 8.5.5. Analysis by Type of Expansion and Type of Product
- 8.5.6. Analysis by Area of Expansion
- 8.5.7. Most Active Players: Analysis by Number of Expansions
- 8.5.8. Analysis by Region
- 8.5.8.1. Analysis by Location of Facility (Continent-wise)
- 8.5.8.2. Analysis by Location of Facility (Country-wise)
- 8.5.9. Analysis by Type of Expansion and Location of Facility
9. LIKELY PARTNER ANALYSIS FOR CELL THERAPY CONSUMABLE PROVIDERS
- 9.1. Chapter Overview
- 9.2. Scoring Criteria and Key Assumptions
- 9.3. Scope and Methodology
- 9.4. Key Potential Strategic Partners for Cell Therapy Consumable Providers
- 9.4.1. Likely Partners for Dendritic Cell Therapy Consumable Providers
- 9.4.2. Likely Partners for NK Cell Therapy Consumable Providers
- 9.4.3. Likely Partners for Stem Cell Therapy Consumable Providers
- 9.4.4. Likely Partners for T-Cell Therapy Consumable Providers
10. ROOTS ANALYSIS PRICING STRATEGY
- 10.1. Chapter Overview
- 10.2. Roots Analysis Pricing Strategy Framework
- 10.2.1. Theoretical Framework and Price Evaluation Hypothesis for Cell Therapy Media
- 10.2.1.1. Methodology
- 10.2.1.2. Results and Interpretation
- 10.2.1.2.1. Cell Therapy Media Price Evaluation Matrix: Information on Volume of Media
- 10.2.1.2.2. Cell Therapy Media Price Evaluation Matrix: Information on Media Compatibility
- 10.2.1.2.3. Cell Therapy Media Price Evaluation Matrix: Information on Type of Product Manufacturing Practices
- 10.2.1.2.4. Cell Therapy Media Price Evaluation Matrix: Information on Application Area
- 10.2.1.2.5. Cell Therapy Media Price Evaluation Matrix: Information on Storage Temperature
- 10.2.1.2.6. Cell Therapy Media Price Evaluation Matrix: Information on Type of Cell Therapy
- 10.2.1.2.7. Cell Therapy Media Price Evaluation Matrix: Information on Type of Function
- 10.2.2. Theoretical Framework and Price Evaluation Hypothesis of Cell Therapy Extracellular Matrices
- 10.2.2.1. Methodology
- 10.2.2.2. Results and Interpretation
- 10.2.2.2.1. Cell Therapy Extracellular Matrices Price Evaluation Matrix: Information on Type of ECM Coating
- 10.2.2.2.2. Cell Therapy Extracellular Matrices Price Evaluation Matrix: Information on Type of Formulation
- 10.2.2.2.3. Cell Therapy Extracellular Matrices Price Evaluation Matrix: Information on Volume of Extracellular Matrices
- 10.2.2.2.4. Cell Therapy Extracellular Matrices Price Evaluation Matrix: Information on Storage Temperature
- 10.2.2.2.5. Cell Therapy Extracellular Matrices Price Evaluation Matrix: Information on Shelf Life
- 10.2.2.2.6. Cell Therapy Extracellular Matrices Price Evaluation Matrix: Information on Type of Stem Cell Therapy
- 10.2.2.2.7. Cell Therapy Extracellular Matrices Price Evaluation Matrix: Information on Type of Function
- 10.2.1. Theoretical Framework and Price Evaluation Hypothesis for Cell Therapy Media
11. DEMAND ANALYSIS
- 11.1. Chapter Overview
- 11.2. Scope and Methodology
- 11.3. Global Demand for Cell Therapy Consumables
- 11.3.1. Global Demand for Cell Therapy Consumables for Planar Processes
- 11.3.2. Global Demand for Cell Therapy Consumables for Suspension Processes
- 11.4. Analysis by Scale of Operation
- 11.5. Analysis by Geography
12. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 12.1. Chapter Overview
- 12.2. Key Assumptions and Methodology
- 12.3. Global Cell Therapy Consumables Market, Till 2035
- 12.4. Cell Therapy Consumables Market: Analysis by Type of Product
- 12.4.1. Cell Therapy Consumables Market for Extracellular Matrices, Till 2035
- 12.4.2. Cell Therapy Consumables Market for Kits, Till 2035
- 12.4.3. Cell Therapy Consumables Market for Media, Till 2035
- 12.4.4. Cell Therapy Consumables Market for Reagents, Till 2035
- 12.5. Cell Therapy Consumables Market: Analysis by Type of Cell Therapy
- 12.5.1. Cell Therapy Consumables Market for Dendritic Cell Therapies, Till 2035
- 12.5.2. Cell Therapy Consumables Market for NK Cell Therapies, Till 2035
- 12.5.3. Cell Therapy Consumables Market for Stem Cell Therapies, Till 2035
- 12.5.4. Cell Therapy Consumables Market for T-Cell Therapies, Till 2035
- 12.6. Cell Therapy Consumables Market: Analysis by Scale of Operation
- 12.6.1. Cell Therapy Consumables Market for Clinical Operations, Till 2035
- 12.6.2. Cell Therapy Consumables Market for Commercial Operations, Till 2035
- 12.7. Cell Therapy Consumables Market: Analysis by Type of End-User
- 12.7.1. Cell Therapy Consumables Market for Industry Players, Till 2035
- 12.7.2. Cell Therapy Consumables Market for Non-Industry Players, Till 2035
- 12.8. Cell Therapy Consumables Market: Analysis by Geography
- 12.8.1. Cell Therapy Consumables Market in North America, Till 2035
- 12.8.1.1. Cell Therapy Consumables Market in the US, Till 2035
- 12.8.1.2. Cell Therapy Consumables Market in Canada, Till 2035
- 12.8.1.3. Cell Therapy Consumables Market in Rest of North America, Till 2035
- 12.8.2. Cell Therapy Consumables Market in Europe, Till 2035
- 12.8.2.1. Cell Therapy Consumables Market in Spain, Till 2035
- 12.8.2.2. Cell Therapy Consumables Market in France, Till 2035
- 12.8.2.3. Cell Therapy Consumables Market in Germany, Till 2035
- 12.8.2.4. Cell Therapy Consumables Market in Italy, Till 2035
- 12.8.2.5. Cell Therapy Consumables Market in the Netherlands, Till 2035
- 12.8.2.6. Cell Therapy Consumables Market in the UK, Till 2035
- 12.8.2.7. Cell Therapy Consumables Market in Rest of Europe, Till 2035
- 12.8.3. Cell Therapy Consumables Market in Asia-Pacific, Till 2035
- 12.8.3.1. Cell Therapy Consumables Market in China, Till 2035
- 12.8.3.2. Cell Therapy Consumables Market in Korea, Till 2035
- 12.8.3.3. Cell Therapy Consumables Market in Rest of Asia-Pacific, Till 2035
- 12.8.4. Cell Therapy Consumables Market in Middle East and North Africa, Till 2035
- 12.8.5. Cell Therapy Consumables Market in Latin America, Till 2035
- 12.8.1. Cell Therapy Consumables Market in North America, Till 2035
13. UPCOMING TRENDS AND FUTURE GROWTH OPPORTUNITIES
- 13.1. Chapter Overview
- 13.2. Emerging Trends Related to Cell Culture Media
- 13.3. Automation of Cell Therapy Manufacturing Processes
- 13.4. Single Use Systems and Technologies in Cell Therapy Manufacturing
14. CONCLUDING REMARKS
15. INTERVIEW TRANSCRIPTS
- 15.1. Chapter Overview
- 15.2. Company A
- 15.2.1. Interview Transcript: Vice President of Business Development - Cell Therapy
- 15.3. Company B
- 15.3.1. Interview Transcript: Chief Operating Officer
- 15.4. Company C
- 15.4.1. Interview Transcript: Director R&D, Cell Culture and Immunology and, Assistant R&D Manager, Animal Cell Culture



















