
|
시장보고서
상품코드
1762531
프리필드 시린지 Fill Finish 제조 시장 : 업계 동향과 세계 예측 - 치료 영역별, 시린지 배럴 재질별, 약제 분자별, 사업 규모별, 주요 지역별Prefilled Syringe Fill Finish Manufacturing Market: Industry Trends and Global Forecasts - Distribution by Type of Therapeutic Area, Type of Syringe Barrel Material, Type of Drug Molecule, Scale of Operation and Key Geographical Regions |
||||||
프리필드 시린지 Fill Finish 제조 시장 : 개요
세계의 프리필드 시린지 Fill Finish 제조 시장 규모는 올해 9억 3,100만 달러에 달했습니다. 이 시장은 예측 기간 중 11.9%의 유리한 CAGR로 성장할 것으로 예측됩니다.
시장 규모 및 기회 분석은 다음과 같은 매개 변수에 걸쳐 세분화되어 있습니다.
치료 영역
- 혈액 질환
- 감염성 질환
- 대사성 질환
- 종양 질환
- 신경질환
- 자가면역질환
- 기타
주사기 배럴 재질
- 유리
- 플라스틱
배럴 챔버 수
- 싱글 챔버
- 듀얼 챔버
약제 분자
- 생물제제
- 저분자
사업 규모
- 상업
- 전임상/임상
주요 지역
- 북미
- 유럽
- 아시아태평양
프리필드 시린지 Fill Finish 제조 시장 : 성장과 동향
기본적으로 프리필드 시린지는 미리 측정된 양의 주사약을 담은 1회분 약품입니다. 주사제 및 백신의 주요 약품 용기로 사용됩니다. 프리필드 시린지에 채워지는 약물의 범주에는 혈액 자극제, 에리스로포이에틴 제제, 인터페론, 치료용 단백질 등이 있습니다. 지난 10년동안 비경구 의약품의 개발이 증가함에 따라 프리필드 시린지 사용량이 3 배 증가했습니다. 특히 이러한 프리필드 시린지는 기존의 약물전달 시스템(바이알, 주사기 등)에 비해 투약 실수 가능성 감소, 환자 순응도 향상, 미생물 오염 위험 감소 등 여러 가지 이점을 제공합니다. 그 결과, 프리필드 시린지는 주사제 전달, 특히 만성질환(반복적인 약물 투여가 필요한)의 치료에 선호되고 있습니다.
프리필드 시린지가 제공하는 여러 가지 장점은 셀프 메디케이션 증가 추세와 함께 이러한 장비와 결합된 많은 의약품의 승인으로 이어졌고, 그 결과 충전/마무리 작업의 아웃소싱에 대한 필요성이 증가하고 있습니다. 또한 주사기 충전량의 면밀한 모니터링 및 관리에 따른 기술적 전문 지식의 필요성 등 여러 가지 업무상의 문제로 인해 시장에서는 프리필드 주사기 충전 및 마감 작업의 아웃소싱으로 전환하는 추세가 나타나고 있습니다. 또한 보다 안전하고 사용하기 쉬운 투약 기술에 대한 현재 수요와 비용 절감 및 효율성 향상을 위한 업계의 노력은 향후 프리필드 시린지 충전 및 마감 제조 시장의 성장을 가속할 것으로 예측됩니다.
프리필드 시린지 Fill Finish 제조 시장 : 주요 인사이트
이 보고서는 세계 프리필드 시린지 Fill Finish 제조 시장의 현황을 조사하고 업계의 잠재적인 성장 기회를 파악합니다. 이 보고서의 주요 조사 결과는 다음과 같습니다.
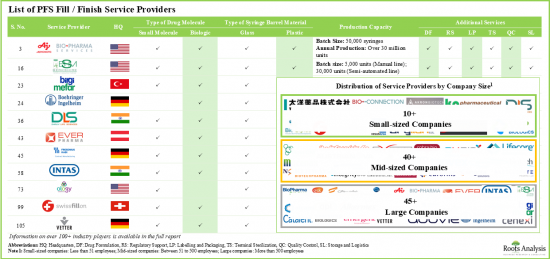
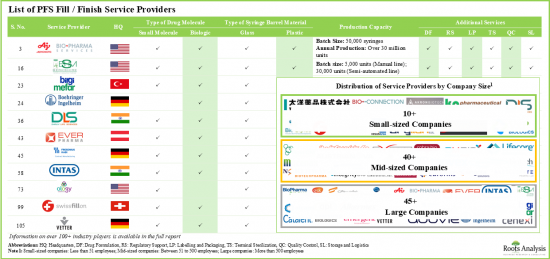
- 전 세계에서 100개 이상의 회사가 프리필드 주사기 위탁 Fill Finish 서비스를 제공한다고 주장하며, 이들 서비스 프로바이더 중 약 50%가 500명 이상의 직원을 보유한 대기업이라고 합니다.
- 시장 상황은 이 분야에서 명성을 쌓은 기업이 존재한다는 특징이 있으며, 그 중 상당수는 다양한 사업 규모로 사업을 전개하고 다양한 지역에 위치하고 있다고 주장하고 있습니다.
- 55% 이상의 기업이 저분자 의약품과 생물제제 모두에 대한 Fill Finish 서비스를 제공하고 있으며, 생물제제만을 대상으로 하는 기업은 20% 정도입니다.
- 공급망 전반의 역량을 확보하고 고객의 진화하는 니즈에 대응하기 위해 다양한 규모의 서비스를 제공하는 기업이 다양한 지역에 진출하고 있습니다.
- AMRI, Catalent Biologics, Pierre Fabrre, Sandoz 등 여러 위탁 서비스 프로바이더(약 60개사)가 임상 및 상업적 규모 모두에서 충전 및 마무리 서비스를 제공합니다.
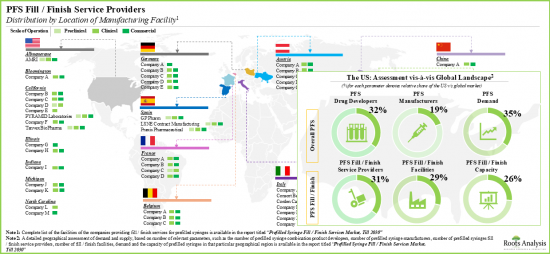
- 업계 이해관계자 대부분이 선진국에 기반을 두고 있지만, 인도, 대만, 방글라데시 등 아시아 국가들의 충진/마무리 사업이 부상하고 있습니다.
- 충전/마무리 서비스 프로바이더는 기존 용량과 생산 능력을 업그레이드하기 위해 확장 프로젝트에 적극적으로 투자하고 있습니다.
- 예상대로, 프리필드 시린지용 충진/마무리 위탁 장비의 대부분은 대형 서비스 프로바이더에 속해 있으며, 전 세계 사용 가능한 장비의 약 80%를 차지하고 있습니다.
- 프리필드 주사기 조합 제품 개발자를 평가한 결과, 유럽과 북미가 충전/마무리 서비스 프로바이더에게 중요한 협력 기회가 있는 지역으로 밝혀졌습니다.
- 약 70여 개의 프리필드 시린지가 주요 기업에서 생산되고 있으며, 대부분 유리와 플라스틱 재료로 주사기 배럴을 생산할 수 있다고 주장하고 있습니다.
- 단-중기적으로 의약품 개발 기업별 충진 마무리 업무의 아웃소싱이 지속되어 위탁 서비스 시장의 연평균 성장률은 11.9%를 상회할 것으로 예측됩니다.
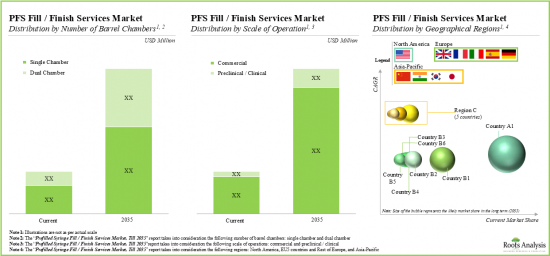
- 장기적으로 볼 때, 프리필드 시린지 충전 및 마감 서비스 프로바이더에게 예상되는 기회는 다양한 유형의 배럴 챔버, 사업 규모 및 지역적 지역에 잘 분산되어 있을 것으로 보입니다.
- 프리필드 시린지에 대한 수요 증가와 사용자 친화적인 시스템에 대한 관심으로 인해 향후 수년간 시장 기회는 빠른 속도로 발전할 것으로 예측됩니다.
프리필드 시린지 Fill Finish 제조 시장의 참여 기업 예
- Ajinomoto Bio-Pharma Services
- AMRI
- BioPharma Solutions
- Emergent BioSolutions
- Patheon(a Thermo Fisher Scientific Company)
- Consort Medical
- EVER Pharma
- GlaxoSmithKline
- IDT Biologika
- Rentschler Biopharma
- Siegfried
- Vetter Pharma
- Biocon
- Intas Pharmaceuticals
- Kemwell Biopharma
- Square Pharmaceuticals.
목차
제1장 서문
제2장 개요
제3장 서론
- 챕터 개요
- 프리필드 시린지의 서론
- 프리필드 시린지의 Fill Finish 처리
- 프리필드 시린지 Fill Finish 시장의 성장요인
- 멸균 주사제의 Fill Finish 업무 아웃소싱의 필요성
- Fill Finish 서비스 아웃소싱의 이점
- Fill Finish 업무의 아웃소싱에 수반하는 리스크
- Fill Finish 서비스 프로바이더 선택시 주요 고려 사항
제4장 시장 구도
- 챕터 개요
- 프리필드 시린지 Fill Finish 서비스 프로바이더 : 시장 구도
제5장 북미의 프리필드 시린지 Fill Finish 서비스 프로바이더 : 기업 개요
- 챕터 개요
- Ajinomoto Bio-Pharma Services
- AMRI
- BioPharma Solutions
- Emergent BioSolutions
- Patheon
제6장 유럽의 프리필드 시린지 Fill Finish 서비스 프로바이더 : 기업 개요
- 챕터 개요
- Consort Medical
- EVER Pharma
- GlaxoSmithKline
- IDT Biologika
- Rentschler Biopharma
- Siegfried
- Vetter Pharma
제7장 아시아태평양의 프리필드 시린지 Fill Finish 서비스 프로바이더 : 기업 개요
- 챕터 개요
- Biocon
- Intas Pharmaceuticals
- Kemwell Biopharma
- Square Pharmaceuticals
제8장 프리필드 시린지 Fill Finish 서비스 프로바이더 : 최근 동향
- 챕터 개요
- 확장 리스트
- 파트너십 모델
- 파트너십과 협업 리스트
제9장 용량 분석
제10장 수요 분석
제11장 수요와 공급의 지역적 평가
- 챕터 개요
- 전제와 주요 파라미터
- 북미에서 수요와 공급의 평가
- 유럽에서 수요와 공급의 평가
- 아시아태평양에서 수요와 공급의 평가
제12장 가능성 있는 파트너 분석
제13장 시장 규모의 평가와 기회 분석
- 챕터 개요
- 예측 조사 방법과 주요 전제조건
- 프리필드 시린지 Fill Finish 서비스 시장 전체(-2035년)
제14장 향후 성장 기회
- 챕터 개요
- 성장하는 주사제 파이프라인
- 프리필드 시린지의 인기 증가
- Fill Finish 업무의 아웃소싱에 대한 선호도의 증가
- 무균 Fill Finish 기술의 진보
- 아시아태평양에서 기회의 확대
제15장 사례 연구 : 프리필드 시린지 제조업체
- 챕터 개요
- 프리필드 시린지 : 입수 가능/개발중인 디바이스 리스트
- 프리필드 시린지 : 제조업체 리스트
제16장 결론
제17장 인터뷰 기록
제18장 부록 1 : 표형식 데이터
제19장 부록 2 : 기업 리스트
KSA 25.07.10PREFILLED SYRINGE FILL FINISH MANUFACTURING MARKET: OVERVIEW
As per Roots Analysis, the global prefilled syringe fill finish manufacturing market valued at USD 931 million in the current year is anticipated to grow at a lucrative CAGR of 11.9% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Therapeutic Area
- Blood Disorders
- Infectious Diseases
- Metabolic Disorders
- Oncology Disorders
- Neurological Disorders
- Autoimmune Disorders
- Others
Type of Syringe Barrel Material
- Glass
- Plastic
Number of Barrel Chambers
- Single Chamber
- Dual Chamber
Type of Drug Molecule
- Biologic
- Small Molecule
Scale of Operation
- Commercial
- Preclinical / Clinical
Key Geographical Regions
- North America
- Europe
- Asia- Pacific
PREFILLED SYRINGE FILL FINISH MANUFACTURING MARKET: GROWTH AND TRENDS
Fundamentally, a prefilled syringe is a single dose of medication, containing a pre-measured amount of injectable. It is used as a primary drug container for injectable drugs and vaccines. Some of the categories of drugs packaged in prefilled syringes include blood stimulants, erythropoietin products, interferons and therapeutic proteins. Over the past ten years, with the increase in development of parenteral drugs, the usage of prefilled syringes has tripled. Notably, these prefilled syringes offer several advantages over traditional drug delivery systems (such as vials and syringes), including reduced chances of dosing errors, increased patient compliance and decreased risk of microbial contamination. As a result, prefilled syringes are preferably being used in the delivery of injectable drugs, especially in the treatment of chronic diseases (requiring repeated administration of the medications).
The numerous benefits offered by prefilled syringes, coupled with the growing self-medication trend, have led to the approval of a number of drugs in combination with such devices, resulting in an increase in need to outsource fill / finish operations. Moreover, owing to a number of operational challenges, such as the requirement of technical expertise associated with close monitoring and control of syringe fill volume, the market has witnessed a shift in trend towards outsourcing of the fill / finish operations for prefilled syringes. Additionally, the current demand for safer, easier to use administration technologies along with the industry's efforts to reduce costs and increase efficiency are expected to drive the growth of the prefilled syringe fill finish manufacturing market in future.
PREFILLED SYRINGE FILL FINISH MANUFACTURING MARKET: KEY INSIGHTS
The report delves into the current state of the global prefilled syringe fill finish manufacturing market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Over 100 companies across the globe claim to provide contract fill / finish services for prefilled syringes; close to 50% of these service providers are large companies with over 500 employees.
- The market landscape features the presence of well-established players in the domain, most of which claim to operate at various scales of operation and are located in different geographies.
- Over 55% of the companies have the capabilities to offer fill / finish services for both small molecules and biologics; ~20% of the companies are offering these services for only biologics.
- In order to acquire competencies across the supply chain and cater to the evolving needs of clients, companies offering services across different scales of operation have established presence in various geographies.
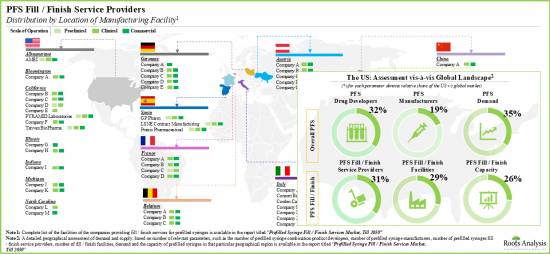
- Several contract service providers (~60), including AMRI, Catalent Biologics, Pierre Fabrre and Sandoz offer fill / finish services at both clinical and commercial scale of operation.
- Although most industry stakeholders are based in the developed regions, fill / finish operations in Asian countries, such as India, Taiwan and Bangladesh, are emerging.
- Fill / finish service providers are actively investing in expansion projects to upgrade existing capabilities and capacity; several partnerships, mostly focused on offering contract services, have also been forged.
- As expected, majority of the installed contract fill / finish capacity for prefilled syringes belongs to the large service providers, accounting for around 80% of the available global capacity.
- An evaluation of prefilled syringe combination product developers revealed that Europe and North America have emerged as the key regions for partnering opportunities for fill / finish service providers.
- Around 70 prefilled syringes are being manufactured by large companies, most of which claim to be capable of fabricating syringe barrels from both glass and plastic materials.
- We expect drug developers to continue to outsource their fill / finish operations in the short to midterm, resulting in an annualized growth at the rate of over 11.9% within the contract services market.
- In the long-term, the projected opportunity for the fill / finish service providers for prefilled syringes is likely to be well distributed across various types of barrel chambers, scales of operation and geographical regions.
- With an increasing demand for prefilled syringes and focus on user-friendly systems, the market opportunity is projected to evolve at a rapid pace in the coming years.
Example Players in the Prefilled Syringe Fill Finish Manufacturing Market
- Ajinomoto Bio-Pharma Services
- AMRI
- BioPharma Solutions
- Emergent BioSolutions
- Patheon (a Thermo Fisher Scientific Company)
- Consort Medical
- EVER Pharma
- GlaxoSmithKline
- IDT Biologika
- Rentschler Biopharma
- Siegfried
- Vetter Pharma
- Biocon
- Intas Pharmaceuticals
- Kemwell Biopharma
- Square Pharmaceuticals.
PREFILLED SYRINGE FILL FINISH MANUFACTURING MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the prefilled syringe fill finish manufacturing market, focusing on key market segments, including [A] type of therapeutic area, [B] type of syringe barrel material, [C] number of barrel chambers, [D] type of drug molecule, [E] scale of operation and [F] key geographical regions.
- Market Landscape: A comprehensive evaluation of companies offering contract fill / finish services for prefilled syringes, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] scale of operation, [D] location of the headquarters, [E] location of fill / finish facilities, [F] type of drug molecule, [G] syringe barrel material, [H] syringe fill volume and additional services offered and [I] installed capacity.
- Company Profiles: In-depth profiles of the players engaged in this domain, focusing on [A] overview of the company, [B] financial information (if available), [C] service portfolio, [D] fill / finish facilities and [E] recent developments and an informed future outlook.
- Recent Developments: A comprehensive competitive analysis of recent developments pertaining to contract fill / finish services, based on various parameters, such as [A] year of development, [B] type of activity / development, [C] scale of operation of the project, [D] location of expanded facility, [E] type of drug molecule involved and [F] additional services offered.
- Capacity Analysis: An in-depth analysis of contract fill / finish capacity of prefilled syringes, examining factors, such as [A] company size of manufacturer, [B] scale of operation, [C] location of headquarters and fill / finish facilities and [D] type of drug molecule.
- Demand Analysis: A detailed analysis of the current and future demand for fill / finish of prefilled syringes, based on various parameters, such as [A] marketed drugs available, [B] target patient population, [C] dosing frequency, [D] dose strength, [E] geography, [F] type of drug molecule, [G] therapeutic area, [H] syringe barrel material and [I] number of barrel chambers.
- Geographical Assessment of Demand and Supply: An insightful geographical analysis of demand and supply, based on several parameters, such as [A] number of prefilled syringe combination product developers, [B] number of prefilled syringe manufacturers, [C] number of prefilled syringe fill / finish service providers, [D] number of prefilled syringe fill / finish facilities, and [E] capacity and demand for prefilled syringes in that particular geographical region.
- Likely Partner Analysis: A detailed analysis of the potential strategic partners for prefilled syringe fill / finish service providers, based on various parameters, such as [A] developer strength, [B] pipeline strength, [C] type of drug molecule, [D] target therapeutic area and [E] location of the headquarters of the company.
- Case Study 1: A detailed discussion on the potential areas of growth, such as [A] growing injectable drugs pipeline, [B] increasing popularity of prefilled syringes, [C] rise in preference for outsourcing of fill / finish operations, [D] technological advancements in aseptic fill / finish processes and [E] growing opportunities in the Asia-Pacific region.
- Case Study 2: Elaborate assessment of prefilled syringe manufacturers landscape, based on a number of relevant parameters, such as [A] syringe barrel material, [B] number of barrel chambers, [C] type of needle system, [D] barrel volume, [E] year of establishment and [F] location of the headquarters of manufacturers.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Introduction to Prefilled Syringes
- 3.2.1. Classification of Prefilled Syringes
- 3.2.2. Manufacturing of Prefilled Syringes
- 3.2.3. Advantages of Prefilled Syringes
- 3.2.3.1. Benefits to Healthcare Professionals and End Users
- 3.2.3.2. Benefits to Manufacturers
- 3.3. Fill / Finish Processing of Prefilled Syringes
- 3.3.1. Steps Involved in Fill / Finish Process
- 3.3.2. Methods of Filling and Stoppering of Prefilled Syringes
- 3.3.3. Prefilled Syringe Filling Technologies
- 3.4. Factors Contributing to the Growth of Prefilled Syringe Fill / Finish Market
- 3.5. Need for Outsourcing Fill / Finish Operations of Sterile Injectables
- 3.6. Advantages of Outsourcing Fill / Finish Services
- 3.7. Risks Associated with Outsourcing of Fill / Finish Operations
- 3.8. Key Considerations While Selecting a Fill / Finish Service Provider
4. MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Prefilled Syringe Fill / Finish Service Providers: Overall Market Landscape
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Scale of Operation
- 4.2.4. Analysis by Location of Headquarters
- 4.2.5. Analysis by Location of Prefilled Syringe Fill / Finish Facilities
- 4.2.6. Analysis by Type of Drug Molecule
- 4.2.7. Analysis by Syringe Barrel Material
- 4.2.8. Analysis by Syringe Fill Volume
- 4.2.9. Analysis by Additional Services Offered
- 4.2.10. Heat Map: Analysis by Company Size and Location of Headquarters
- 4.2.11. Logo Landscape: Analysis by Company Size and Type of Drug Molecule
- 4.2.12. Geographical Map: Analysis by Scale of Operation and Location of Fill / Finish Facilities
- 4.2.13. Grid Representation: Distribution by Year of Establishment, Company Size and Type of Drug Molecule
5. PREFILLED SYRINGE FILL / FINISH SERVICE PROVIDERS IN NORTH AMERICA: COMPANY PROFILES
- 5.1. Chapter Overview
- 5.2. Ajinomoto Bio-Pharma Services
- 5.2.1. Company Overview
- 5.2.2. Recent Developments and Future Outlook
- 5.3. AMRI
- 5.3.1. Company Overview
- 5.3.2. Recent Developments and Future Outlook
- 5.4. BioPharma Solutions
- 5.4.1. Company Overview
- 5.4.2. Recent Developments and Future Outlook
- 5.5. Emergent BioSolutions
- 5.5.1. Company Overview
- 5.5.2. Recent Developments and Future Outlook
- 5.6. Patheon (a Thermo Fisher Scientific Company)
- 5.6.1. Company Overview
- 5.6.2. Recent Developments and Future Outlook
6. PREFILLED SYRINGE FILL / FINISH SERVICE PROVIDERS IN EUROPE: COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. Consort Medical
- 6.2.1. Company Overview
- 6.2.2. Recent Developments and Future Outlook
- 6.3. EVER Pharma
- 6.3.1. Company Overview
- 6.3.2. Recent Developments and Future Outlook
- 6.4. GlaxoSmithKline
- 6.4.1. Company Overview
- 6.4.2. Recent Developments and Future Outlook
- 6.5. IDT Biologika
- 6.5.1. Company Overview
- 6.5.2. Recent Developments and Future Outlook
- 6.6. Rentschler Biopharma
- 6.6.1. Company Overview
- 6.6.2. Recent Developments and Future Outlook
- 6.7. Siegfried
- 6.7.1. Company Overview
- 6.7.2. Recent Developments and Future Outlook
- 6.8. Vetter Pharma
- 6.8.1. Company Overview
- 6.8.2. Recent Developments and Future Outlook
7. PREFILLED SYRINGE FILL / FINISH SERVICE PROVIDERS IN ASIA-PACIFIC: COMPANY PROFILES
- 7.1. Chapter Overview
- 7.2. Biocon
- 7.2.1. Company Overview
- 7.2.2. Recent Developments and Future Outlook
- 7.3. Intas Pharmaceuticals
- 7.3.1. Company Overview
- 7.3.2. Recent Developments and Future Outlook
- 7.4. Kemwell Biopharma
- 7.4.1. Company Overview
- 7.4.2. Recent Developments and Future Outlook
- 7.5. Square Pharmaceuticals
- 7.5.1. Company Overview
- 7.5.2. Recent Developments and Future Outlook
8. PREFILLED SYRINGE FILL / FINISH SERVICE PROVIDERS: RECENT DEVELOPMENTS
- 8.1. Chapter Overview
- 8.2. List of Expansions
- 8.2.1. Analysis by Year of Expansion
- 8.2.2. Analysis by Type of Expansion
- 8.2.3. Analysis by Expanded Scale of Operation
- 8.2.4. Analysis by Location of Expansion Project
- 8.2.5. Analysis by Type of Drug Molecule Involved
- 8.2.6. Analysis by Additional Services Offered (Expansion Specific)
- 8.3. Partnership Models
- 8.4. List of Partnerships and Collaborations
- 8.4.1. Analysis by Year of Partnership
- 8.4.2. Analysis by Type of Partnership
- 8.4.3. Analysis by Scale of Operation (Deal Specific)
- 8.4.4. Analysis by Additional Services Offered (Deal Specific)
9. CAPACITY ANALYSIS
- 9.1. Chapter Overview
- 9.2. Assumptions and Methodology
- 9.3. Global, Prefilled Syringe Fill / Finish Capacity (by Number of Units)
- 9.3.1. Analysis by Company Size
- 9.3.2. Analysis by Scale of Operation
- 9.3.3. Analysis by Location of Headquarters
- 9.3.4. Analysis by Location of Fill / Finish Facilities
- 9.3.5. Analysis by Type of Drug Molecule
- 9.4. Global, Prefilled Syringe Fill / Finish Capacity (by Volume)
- 9.4.1. Analysis by Company Size (Manufacturer)
- 9.4.2. Analysis by Scale of Operation
- 9.4.3. Analysis by Location of Headquarters
- 9.4.4. Analysis by Location of Fill / Finish Facilities
- 9.4.5. Analysis by Type of Drug Molecule
10. DEMAND ANALYSIS
- 10.1. Chapter Overview
- 10.2. Assumptions and Methodology
- 10.3. Global Demand for Prefilled Syringes Fill / Finish Services
- 10.3.1. Global Demand for Commercialized Prefilled Syringes Fill / Finish Services
- 10.3.1.1. Analysis by Geography
- 10.3.1.1.1. North America
- 10.3.1.1.2. Europe
- 10.3.1.1.3. Asia-Pacific
- 10.3.1.1.4. Latin America
- 10.3.1.1.5. Middle East and Africa
- 10.3.1.2. Analysis by Syringe Barrel Material
- 10.3.1.3. Analysis by Number of Barrel Chambers
- 10.3.1.4. Analysis by Type of Drug Molecule
- 10.3.1.5. Analysis by Therapeutic Area
- 10.3.1.6. Analysis by Specialty Syringes
- 10.3.1.1. Analysis by Geography
- 10.3.2. Global Demand for Clinical Prefilled Syringes Fill / Finish Services
- 10.3.1. Global Demand for Commercialized Prefilled Syringes Fill / Finish Services
- 10.4. Demand and Supply Analysis
- 10.4.1. Demand and Supply Analysis (Scenario 1)
- 10.4.2. Demand and Supply Analysis (Scenario 2)
- 10.4.3. Demand and Supply Analysis (Scenario 3)
11. GEOGRAPHICAL ASSESSMENT OF DEMAND AND SUPPLY
- 11.1. Chapter Overview
- 11.2. Assumptions and Key Parameters
- 11.3. Demand and Supply Assessment in North America
- 11.3.1. Key Geographies
- 11.3.1.1. US
- 11.3.1. Key Geographies
- 11.4. Demand and Supply Assessment in Europe
- 11.4.1. Key Geographies
- 11.4.1.1. France
- 11.4.1.2. Germany
- 11.4.1.3. Italy
- 11.4.1. Key Geographies
- 11.5. Demand and Supply Assessment in Asia-Pacific Region
- 11.5.1. Key Geographies
- 11.5.1.1. India
- 11.5.1.2. Japan
- 11.5.1. Key Geographies
12. LIKELY PARTNER ANALYSIS
- 12.1. Chapter Overview
- 12.2. Methodology and Key Parameters
- 12.3. Likely Partner Analysis
- 12.3.1. Opportunities in North America
- 12.3.1.1. Most Likely Partners for Prefilled Syringe Fill / Finish Service Providers
- 12.3.1.2. Likely Partners for Prefilled Syringe Fill / Finish Service Providers
- 12.3.2. Opportunities in Europe
- 12.3.2.1. Most Likely Partners for Prefilled Syringe Fill / Finish Service Providers
- 12.3.2.2. Likely Partners for Prefilled Syringe Fill / Finish Service Providers
- 12.3.3. Opportunities in Asia-Pacific and Rest of the World
- 12.3.3.1. Most Likely Partners for Prefilled Syringe Fill / Finish Service Providers
- 12.3.3.2. Likely Partners for Prefilled Syringe Fill / Finish Service Providers
- 12.3.1. Opportunities in North America
13. MARKET SIZING AND OPPORTUNITY ANALYSIS
- 13.1. Chapter Overview
- 13.2. Forecast Methodology and Key Assumptions
- 13.3. Overall Prefilled Syringe Fill / Finish Services Market, Till 2035
- 13.3.1. Prefilled Syringe Fill / Finish Services Market: Distribution by Scale of Operation, Till 2035
- 13.3.2. Prefilled Syringe Fill / Finish Services Market: Distribution by Geographical Region
- 13.3.2.1. Prefilled Syringe Fill / Finish Services Market in North America
- 13.3.2.2. Prefilled Syringe Fill / Finish Services Market in Europe
- 13.3.2.3. Prefilled Syringe Fill / Finish Services Market in Asia-Pacific
- 13.3.2.4. Prefilled Syringe Fill / Finish Services Market Latin America
- 13.3.2.5. Prefilled Syringe Fill / Finish Services Market in Middle East and Africa
- 13.3.3. Prefilled Syringe Fill / Finish Services Market: Distribution by Type of Drug Molecule, Till 2035
- 13.3.4. Prefilled Syringe Fill / Finish Services Market: Distribution by Therapeutic Area
- 13.3.5. Prefilled Syringe Fill / Finish Services Market: Distribution by Syringe Barrel Material, Till 2035
- 13.3.6. Prefilled Syringe Fill / Finish Services Market: Distribution by Number of Barrel Chambers, Till 2035
14. FUTURE GROWTH OPPORTUNITIES
- 14.1. Chapter Overview
- 14.2. Growing Injectable Drugs Pipeline
- 14.3. Increase in Popularity of Prefilled Syringes
- 14.4. Rise in Preference for Outsourcing Fill / Finish Activities
- 14.5. Advances in Aseptic Fill / Finish Technologies
- 14.6. Growing Opportunities in the Asia-Pacific Region
15. CASE STUDY: PREFILLED SYRINGE MANUFACTURERS
- 15.1. Chapter Overview
- 15.2. Prefilled Syringes: List of Available / Under Development Devices
- 15.2.1. Analysis by Syringe Barrel Material
- 15.2.2. Analysis by Number of Barrel Chambers
- 15.2.3. Analysis by Type of Needle System
- 15.2.4. Analysis by Barrel Volume
- 15.3. Prefilled Syringes: List of Manufacturers
- 15.3.1. Analysis by Year of Establishment
- 15.3.2. Analysis by Location of Headquarters
16. CONCLUDING REMARKS
- 16.1. Chapter Overview
- 16.2. Key Takeaways
17. INTERVIEW TRANSCRIPTS
- 17.1. Chapter Overview
- 17.2. Company A
- 17.2.1. Company Snapshot
- 17.2.2. Interview Transcript: President and Cofounder
- 17.3. Company B
- 17.3.1. Company Snapshot
- 17.3.2. Interview Transcript: Chief Technical Officer
- 17.4. Company C
- 17.4.1. Company Snapshot
- 17.4.2. Interview Transcript: Chief Commercial Officer
- 17.5. Company D
- 17.5.1. Company Snapshot
- 17.5.2. Interview Transcript: Associate Director, Head of Process Sciences Formulation and Fill / Finish
- 17.6. Company E
- 17.6.1. Company Snapshot
- 17.6.2. Interview Transcript: Research Director and Head of Formulation Development
- 17.7. Company F
- 17.7.1. Company Snapshot
- 17.7.2. Interview Transcript: Design Director



















