
|
시장보고서
상품코드
1762540
세포주 특성 평가 및 세포주 개발 시장 : 업계 동향과 세계 예측 - 세포주/발현계 유래별, 세포주의 용도별, 기업 규모별, 주요 지역별Cell Line Characterization and Cell Line Development Market: Industry Trends and Global Forecasts - Distribution by Source of Cell Line / Expression System, Application of Cell Line, Company Size and Key Geographical Regions |
||||||
세포주 특성 평가 및 세포주 개발 시장 : 개요
세계의 세포주 특성 평가 및 세포주 개발 시장 규모는 2035년까지의 예측 기간 중 12.5%의 CAGR로 확대하며, 현재 22억 9,000만 달러에서 2035년까지 83억 8,000만 달러로 성장할 것으로 예측됩니다.
시장 세분화에서는 시장 규모와 기회 분석을 다음과 같은 매개 변수로 구분합니다.
세포주/발현계 유래
- 포유류
- 미생물
- 곤충
- 기타
세포주 용도
- 연구개발
- 바이오매뉴팩처링
기업 규모
- 초대형
- 대규모
- 중규모
- 소규모
주요 지역
- 북미
- 유럽
- 아시아태평양
- 기타 지역
세포주 특성 분석 및 세포주 개발 시장 : 성장과 동향
수년 동안 세포주(배양된 세포 집단)는 바이오 제약 산업에서 매우 중요한 역할을 해왔습니다. 잠재적 의약품 후보물질의 스크리닝, 생물제제 개발, 만성질환의 생물학적 연구, 약물의 독성평가를 위한 귀중한 툴입니다. 또한 개발된 세포주는 그 기원과 역사를 확인하고, 주요 특징과 기능성을 확인하기 위해 특성화 및 인증이 가능합니다. 현재 사용되는 세포주의 대부분은 인간, 마우스, 쥐에서 유래한 것이지만, 다른 포유동물이나 비포유동물 종에서 유래한 것도 있으며, 연구개발에 적용의 폭을 넓히고 있습니다.
또한 시간이 지남에 따라 유전자 편집 기술의 발전으로 세포주 개발에 새로운 길이 열리고 있다는 점은 주목할 만합니다. 그럼에도 불구하고 세포주 개발과 특성 평가는 기술적으로 어렵고 경제적으로도 어려운 일입니다. 그 결과, 의약품 개발 기업은 첨단 기술, 인프라, 전문 지식을 갖춘 위탁 서비스 프로바이더에 대한 의존도가 높아지고 있습니다. 최근 들어 의약품 개발자를 지원하고 세포주 개발 및 바이오 제조의 신속화를 위해 노력하는 첨단 자격을 갖춘 연구수탁기관(CRO)과 제조수탁기관(CMO)이 많이 등장하고 있습니다.
세포주 특성화 및 세포주 개발 시장 : 주요 인사이트
이 보고서는 세포주 특성화 및 세포주 개발 시장의 현황을 조사하고, 업계의 잠재적 성장 기회를 파악합니다. 이 보고서의 주요 조사 결과는 다음과 같습니다.
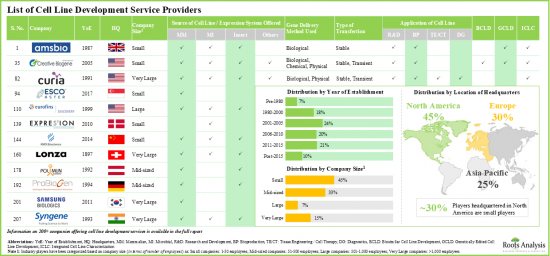
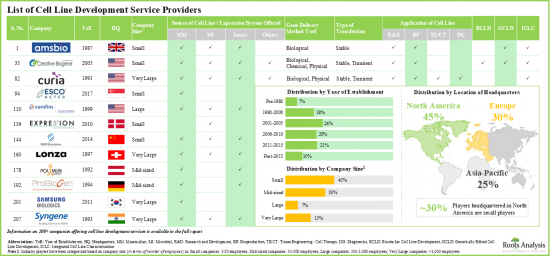
- 현재 200개 이상의 기업(스타트업뿐만 아니라 기존 기업)이 세포주 개발 서비스를 제공하는 데 필요한 역량을 보유하고 있다고 주장하고 있으며, 이들 기업의 대부분은 북미에 기반을 두고 있습니다.
- 이해관계자들은 세포 기반 연구개발 업무를 지원하고, 바이오 치료제 생산을 촉진하는 다양한 출처에서 얻은 세포주를 개발할 수 있는 능력을 가지고 있습니다.
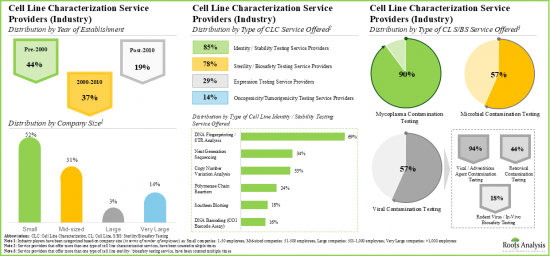
- 140개 이상의 서비스 프로바이더(산업계 및 비산업계)가 세포주 특성화 서비스를 제공한다고 주장하고 있습니다. 이들 중 상당수가 세포주의 동일성과 안정성을 확인하기 위한 유전형질 분석 서비스를 제공합니다.
- 대부분의 세포주 특성평가 서비스 프로바이더는 2000년 이후 설립된 중소기업이 대부분입니다.
- 서비스 프로바이더들은 세포주를 이용해 개발되는 새로운 생물제제에 대한 수요 증가에 대응하기 위해 기술 전문성을 향상시키고, 새로운 역량을 추가하여 서비스 포트폴리오를 강화하고 있습니다.
- 기존 기업도 신규 진출기업도 전략적 파트너십을 맺고 있으며, 가장 두드러진 파트너십 모델로 라이선싱 계약이 부상하고 있습니다.
- 세포주 수요 증가에 대응하기 위해 각 업체들은 시설과 역량을 확대하기 위해 많은 투자를 하고 있습니다. 이러한 추세는 미국과 중국에서 두드러지게 나타나고 있습니다.
- 연구 진전의 원동력이 되는 요인과 연구 진전을 지연시키는 장애요인을 파악하는 것은 전략적 계획의 개선과 효율적인 운영으로 이어집니다.
- 장기적으로 세포주 개발 서비스 시장은 12.5%의 성장이 예상되며, 그 기회는 세포주 공급원, 용도, 지역별로 잘 분산될 것으로 보입니다.
- 제약사 및 연구자들이 세포주 특성평가 업무의 아웃소싱을 계속하고 있으며, 세포주 특성평가 시장은 향후 10년간 연평균 10% 이상 성장할 것으로 예측됩니다.
세포주 특성화 및 세포주 개발 시장 : 주요 부문
세포주/발현계 기원별로 시장은 포유류, 미생물, 곤충, 기타로 구분됩니다. 현재 포유류 세포주 부문은 전 세계 세포주 특성화 시장에서 가장 큰 점유율을 차지하고 있습니다. 이러한 추세는 향후 수년간 지속될 것으로 예측됩니다.
세포주 용도별로 시장은 연구개발과 바이오 제조로 구분됩니다. 현재 전 세계 세포주 특성화 및 세포주 개발 시장에서 가장 높은 비중을 차지하는 것은 바이오 제조 분야입니다. 또한 실험연구를 위한 고품질의 진품 세포주에 대한 수요가 증가함에 따라 연구개발에서의 세포주 적용 기회는 향후 확대될 것으로 예측됩니다.
기업 규모에 따라 시장은 초대형, 대기업, 중견, 중소형으로 구분됩니다. 현재 전 세계 세포주 특성화 및 세포주 개발 시장에서 가장 큰 점유율을 차지하고 있는 것은 중견기업 부문입니다. 또한 이 부문은 향후 더 높은 CAGR로 성장할 것으로 예측됩니다.
주요 지역별로 시장은 북미, 유럽, 아시아태평양, 기타 지역으로 구분됩니다. 현재 북미는 전 세계 세포주 특성화 및 세포주 개발 시장을 독점하고 있으며, 가장 큰 매출 점유율을 차지하고 있습니다. 또한 아시아태평양 시장은 향후 더 높은 CAGR로 성장할 가능성이 높습니다.
세포주 특성 평가 및 세포주 개발 시장의 참여 기업 예
- ATUM
- ATZ Labs(a subsidiary of Life Technologies)
- Avance Biosciences
- BioReliance(acquired by Sigma-Aldrich)
- Biovian
- Celonic Group
- Charles River Laboratories
- ChemPartner
- Cleancells
- Creative Biogene
- Curia
- Eurofins BioPharma Product Testing
- FUJIFILM Diosynth Biotechnologies
- Hylabs
- KBI Biopharma
- Kemp Proteins
- KMD Bioscience
- Livogen Pharmed
- Lonza
- Molecular Diagnostic Services
- Mycenax Biotech
- ProBioGen
- Samsung BioLogics
- Sartorius
- SGS Life Sciences
- Syngene International
- Texcell
- TFBS Bioscience
- Thermo Fisher Scientific
- WuXi Advanced Therapies
- WuXi Biologics
목차
제1장 서문
제2장 조사 방법
제3장 경제적 및 기타 프로젝트 특유 고려 사항
제4장 개요
제5장 서론
- 챕터 개요
- 세포배양의 개요
- 세포주의 특성 평가의 개요
- 세포주의 응용
- 세포주에 관련된 주요 우려 사항
- 세포주 관련 업무 아웃소싱의 필요성
- 결론
제6장 세포주 개발 서비스 프로바이더 : 시장 구도
- 챕터 개요
- 세포주 개발 서비스 프로바이더 : 시장 구도
제7장 세포주 개발 서비스 프로바이더 : 기업 경쟁력 분석
- 챕터 개요
- 전제/주요 파라미터
- 조사 방법
- 세포주 개발 서비스 프로바이더 : 기업 경쟁력 분석
제8장 기업 개요 세포주 개발 서비스 프로바이더
- 챕터 개요
- 주요 서비스 프로바이더의 상세한 기업 개요
- ATUM
- Curia
- Fujifilm Diosynth Biotechnologies
- Lonza
- Syngene International
- WuXi Biologics
- 기타 서비스 프로바이더의 기업 개요
- Biovian
- Celonic
- ChemPartner
- Creative Biogene
- KBI Biopharma
- Kemp Proteins
- KMD Bioscience
- Mycenax Biotech
- ProBioGen
- Thermo Fisher Scientific
제9장 세포주 특성 평가 서비스 프로바이더 : 시장 구도
- 챕터 개요
- 세포주 특성 평가 서비스 프로바이더 : 시장 구도
제10장 세포주 특성 평가 서비스 프로바이더 : 기업 경쟁력 분석
- 챕터 개요
- 전제/주요 파라미터
- 조사 방법
- 세포주 특성 평가 서비스 프로바이더 : 기업 경쟁력 분석
제11장 기업 개요 : 세포주 특성 평가 서비스 프로바이더
- 챕터 개요
- 주요 서비스 프로바이더의 상세한 기업 개요
- Charles River Laboratories
- Eurofins BioPharma Product Testing
- Livogen Pharmed
- Molecular Diagnostic Services
- Sartorius
- TFBS Bioscience
- 기타 서비스 프로바이더의 기업 개요
- ATZ Labs(Subsidiary of Life Technologies)
- Avance Biosciences
- BioReliance
- Clean Cells
- hylabs
- Samsung Biologics
- SGS Life Sciences
- Texcell
- WuXi Advanced Therapies
제12장 파트너십과 협업
- 챕터 개요
- 파트너십 모델
- 세포주의 개발과 특성 평가 : 파트너십과 협업
제13장 최근 확장
- 챕터 개요
- 세포주의 개발과 특성 평가 : 최근 확장 리스트
제14장 세포주 리포지토리
- 챕터 개요
- American Type Culture Collection(ATCC)
- Coriell Institute for Medical Research
- European Collection of Authenticated Cell Cultures(ECACC)
- Leibniz Institute DSMZ - German Collection of Microorganisms and Cell Cultures
- National Centre for Cell Science(NCCS)
제15장 규제에 관한 권고와 가이드라인
- 챕터 개요
- 세포주 인증 : 주요 규제기관
- 국제 규제기관이 발행한 가이드라인
- 미국에서 세포주의 특성 평가에 관한 규제 가이드라인
- 유럽에서 세포주의 특성 평가에 관한 규제 가이드라인
- 일본에서 세포주의 특성 평가에 관한 규제 가이드라인
- 결론
제16장 시장 영향 분석 : 촉진요인, 억제요인, 기회, 과제
제17장 세계의 세포주 개발 서비스 시장
- 챕터 개요
- 전제와 조사 방법
- 세계의 세포주 개발 서비스 시장, 역사적 동향(2019년 이후) 및 예측(-2035년)
- 주요 시장 세분화
제18장 세포주 개발 서비스 시장(세포주 유래별)
- 챕터 개요
- 주요 전제와 조사 방법
- 세포주 개발 서비스 시장 : 세포주 유래별
- 데이터 삼각측량과 검증
제19장 세포주 개발 서비스 시장(세포주의 용도별)
- 챕터 개요
- 주요 전제와 조사 방법
- 세포주 개발 서비스 시장 : 세포주의 용도별
- 데이터 삼각측량과 검증
제20장 세포주 개발 서비스 시장(기업 규모별)
- 챕터 개요
- 주요 전제와 조사 방법
- 세포주 개발 서비스 시장 : 기업 규모별
- 데이터 삼각측량과 검증
제21장 세포주 개발 서비스 시장(주요 지역별)
- 챕터 개요
- 주요 전제와 조사 방법
- 세포주 개발 서비스 시장 : 주요 지역별
- 데이터 삼각측량과 검증
제22장 세계의 세포주 특성 평가 서비스 시장
- 챕터 개요
- 전제와 조사 방법
- 세계의 세포주 특성 평가 서비스 시장, 역사적 동향(2019년 이후) 및 예측(-2035년)
- 주요 시장 세분화
제23장 세포주 특성 평가 서비스 시장(세포주 유래별)
- 챕터 개요
- 주요 전제와 조사 방법
- 세포주 특성 평가 서비스 시장 : 세포주 유래별
- 데이터 삼각측량과 검증
제24장 세포주 특성 평가 서비스 시장(세포주의 용도별)
- 챕터 개요
- 주요 전제와 조사 방법
- 세포주 특성 평가 서비스 시장 : 세포주의 용도별
- 데이터 삼각측량과 검증
제25장 세포주 특성 평가 서비스 시장(기업 규모별)
- 챕터 개요
- 주요 전제와 조사 방법
- 세포주 특성 평가 서비스 시장 : 기업 규모별
- 데이터 삼각측량과 검증
제26장 세포주 특성 평가 서비스 시장(주요 지역별)
- 챕터 개요
- 주요 전제와 조사 방법
- 세포주 특성 평가 서비스 시장 : 주요 지역별
- 데이터 삼각측량과 검증
제27장 결론
제28장 이그제큐티브 인사이트
제29장 부록 1 : 표형식 데이터
제30장 부록 2 : 기업·단체 리스트
제31장 부록 3 : 파트너십과 협력 상세
KSA 25.07.10CELL LINE CHARACTERIZATION AND CELL LINE DEVELOPMENT MARKET: OVERVIEW
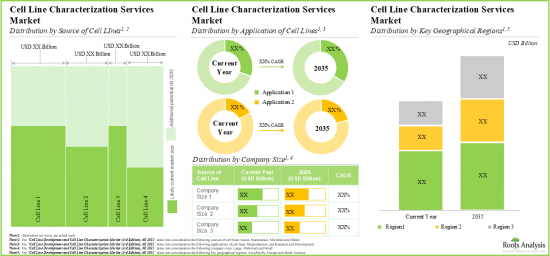
As per Roots Analysis, the global cell line characterization and cell line development market is estimated to grow from USD 2.29 billion in the current year to USD 8.38 billion by 2035, at a CAGR of 12.5% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Source of Cell Line / Expression System
- Mammalian
- Microbial
- Insect
- Others
Application of Cell Line
- Research and Development
- Biomanufacturing
Company Size
- Very Large
- Large
- Mid-sized
- Small
Key Geographical Regions
- North America
- Europe
- Asia Pacific
- Rest of the World
CELL LINE CHARACTERIZATION AND CELL LINE DEVELOPMENT MARKET: GROWTH AND TRENDS
Over the years, cell lines (cultured populations of cells) have become a crucial part of the biopharmaceutical industry. They are a valuable tool for screening potential drug candidates, developing biologics, studying biology of chronic diseases and assessing toxicity of drugs. Moreover, the developed cell lines can be characterized and authenticated to determine their origin / history and identify key characteristics and functionality. While most cell lines in use today are derived from humans, mice, and rats, some also originate from other mammalian and non-mammalian species, broadening their applicability across research and development.
Further, it is worth highlighting that over time the increasing advancements in genome editing technology have opened new avenues for cell line development. Despite this, the development and characterization of cell lines is both technically challenging and financially demanding; as a result, drug developers are becoming increasingly dependent on contract service providers for their advanced technologies, infrastructure and expertise. Recent years have witnessed the emergence of a large number of highly qualified contract research organizations (CROs) and contract manufacturing organizations (CMOs) that assist drug developers and strive to expedite cell line development and biomanufacturing.
CELL LINE CHARACTERIZATION AND CELL LINE DEVELOPMENT MARKET: KEY INSIGHTS
The report delves into the current state of the cell line characterization and cell line development market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Presently, over 200 players (established as well as startups) claim to have the necessary capabilities to offer cell line development services; majority of these firms are based in North America.
- Stakeholders have the capability to develop cell lines obtained from different sources that support cell-based research and development operations and facilitate production of biotherapeutics.
- Over 140 service providers (industry and non-industry) claim to offer cell line characterization services; a sizeable proportion of these players offer genotyping services for accessing the identity and stability of cell lines.
- Majority of the cell line characterization service providers are small and mid-sized firms, established post-2000; notably, around 10% of the players offer all types of characterization services
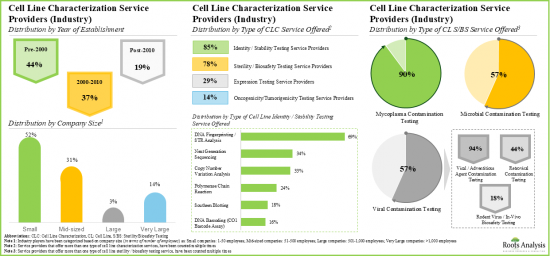
- In order to cater to the rising demand of novel biologics (developed using cell lines), service providers are upgrading their technical expertise and adding new competencies to augment their service portfolios.
- Both well-established players and new entrants have forged strategic partnerships; licensing agreements emerged as the most prominent partnership model.
- To keep pace with the growing demand for cell lines, companies have made significant investments to expand their facilities and capacities; this trend is most pronounced in the US and China.
- Identifying the driving factors (that fuel advancements) as well as barriers (that slow down the research progress) helps improve strategic planning and results in efficient operations.
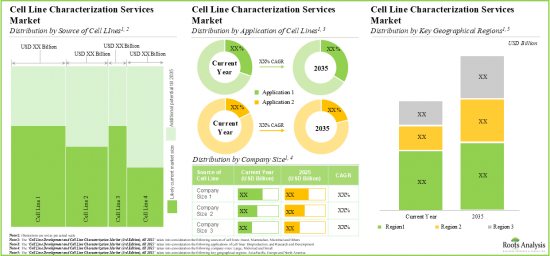
- In the long term, cell line development services market is expected to witness growth of 12.5%; the opportunity is likely to be well distributed across different sources of cell lines, application areas and geographies.
- As pharmaceutical companies and researchers continue outsourcing cell line characterization operations, we anticipate cell line characterization market to grow at an annualized rate of more than 10%, over the next decade.
CELL LINE CHARACTERIZATION AND CELL LINE DEVELOPMENT MARKET: KEY SEGMENTS
Mammalian Cell Line Segment holds the Largest Share of the Global Cell Line Characterization and Cell Line Development Market
Based on the source of cell line / expression system, the market is segmented into mammalian, microbial, insect, and others. At present, the mammalian cell line segment holds the maximum share of the global cell line characterization. This trend is likely to remain the same in the coming years.
By Application of Cell Line, Biomanufacturing is the Fastest Growing Segment of the Global Cell Line Characterization and Cell Line Development Market
Based on the application of cell line, the market is segmented into research and development and biomanufacturing. Currently, the biomanufacturing segment captures the highest proportion of the global cell line characterization and cell line development market. Further due to the growing need for high quality and authentic cell lines for experimental research, the opportunity for cell line application in research and development is anticipated to grow in the future.
Mid-sized Companies Segment Occupy the Largest Share of the Global Cell Line Characterization and Cell Line Development Market
Based on the company size, the market is segmented into very large, large, mid-sized, and small. At present, the mid-sized segment holds the maximum share of the global cell line characterization and cell line development market. Further this segment is expected to grow at a higher CAGR in the coming future.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific and Rest of the World. Currently, North America dominates the global cell line characterization and cell line development market and accounts for the largest revenue share. Further, the market Asia-Pacific is likely to grow at a higher CAGR in the coming future.
Example Players in the Cell Line Characterization and Cell Line Development Market
- ATUM
- ATZ Labs (a subsidiary of Life Technologies)
- Avance Biosciences
- BioReliance (acquired by Sigma-Aldrich)
- Biovian
- Celonic Group
- Charles River Laboratories
- ChemPartner
- Cleancells
- Creative Biogene
- Curia
- Eurofins BioPharma Product Testing
- FUJIFILM Diosynth Biotechnologies
- Hylabs
- KBI Biopharma
- Kemp Proteins
- KMD Bioscience
- Livogen Pharmed
- Lonza
- Molecular Diagnostic Services
- Mycenax Biotech
- ProBioGen
- Samsung BioLogics
- Sartorius
- SGS Life Sciences
- Syngene International
- Texcell
- TFBS Bioscience
- Thermo Fisher Scientific
- WuXi Advanced Therapies
- WuXi Biologics
CELL LINE CHARACTERIZATION AND CELL LINE DEVELOPMENT MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global cell line characterization and cell line development market, focusing on key market segments, including [A] source of cell line / expression system, [B] application of cell line, [C] company size and [D] key geographical regions.
- Cell Line Development Service Providers Market Landscape: A comprehensive evaluation of cell line development service providers, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] source of cell lines / expression systems offered, [E] gene delivery method used, [F] type of transfection, [G] availability of serum free / animal component free culturing capability, [H] type of cells offered, [I] application of cell lines, [J] additional cell line related services offered, [K] type of cell banking, [L] availability of integrated cell line characterization, [M] technology platform utilized, [N] monoclonality procedure used, [O] availability of biosimilar cell line development and [P] gene editing cell line development services.
- Cell Line Development Service Providers Company Competitiveness Analysis: A comprehensive competitive analysis of cell line development service providers, examining factors, such as [A] supplier strength and [B] service strength.
- Company Profiles of Cell Line Development Service Providers: In-depth profiles of key players offering cell line development services, focusing on [A] overview of the company, [B] financial information (if available), [C] service portfolio, [D] recent developments and an informed future outlook. Additionally, in-depth profiles of some other prominent players focusing on [E] overview of the company and [F] cell line development service portfolio.
- Cell Line Characterization Service Providers Market Landscape: A comprehensive evaluation of the companies / organizations providing cell line characterization services, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] source of cell lines and expression systems offered, [E] type of cell line characterization services offered, [F] type of cell identity testing / cell stability testing services offered, [G] type of sterility / biosafety testing services offered, [H] availability of additional cell line related services, [I] regulatory accreditations / certifications and [J] overall turnaround time. Additional information on [K] number of STR loci amplified, [L] type of genotyping kit used, and [M] service fee charged for non-industry players.
- Cell Line Characterization Service Providers Company Competitiveness Analysis: A comprehensive competitive analysis of cell line characterization service providers, examining factors, such as [A] supplier strength and [B] service strength.
- Company Profiles of Cell Line Characterization Service Providers: In-depth profiles of key players offering cell line characterization services, focusing on [A] overview of the company, [B] financial information (if available), [C] cell line characterization service portfolio, [D] recent developments and an informed future outlook. Additionally, in-depth profiles of some other prominent players focusing on [E] overview of the company and [F] cell line characterization service portfolio.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in this domain, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] type of cells involved, [D] therapeutic area, [E] type of partner, [F] most active players (in terms of the number of partnerships signed) and [G] regional distribution of the companies involved in these agreements.
- Recent Expansions: In-depth analysis of the various expansion initiatives undertaken by various cell line development and cell line characterization service providers, based on several parameters, such as [A] year of expansion, [B] type of expansion, [C] location of headquarters, [D] location of expanded facility, [E] area of expanded facility, [F] purpose of expansion, [G] most active players (in terms of number of recent expansions) and [H] geographical distribution.
- Cell Line Repositories: In-depth profiles of the biorepositories across the globe that play an important role in developing cell lines and have also undertaken initiatives to limit the use of contaminated and / or misidentified cell lines, focusing on [A] overview of the repository and [B] cell line characterization service portfolio.
- Regulatory Recommendations and Guidelines: A detailed discussion on the requirements established by various regulatory authorities, across different regions, related to characterization of cell lines. Additionally, a detailed discussion of the various guidelines that have been issued by these bodies related to the protocols that need to be followed while testing of cell lines. Further, a brief description of the historical overview and contributions of key institutes / organizations involved in this domain was represented.
- Market Impact Analysis: A thorough analysis of various factors, such as drivers, restraints, opportunities, and existing challenges that are likely to impact market growth.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Project Objectives
- 1.3. Scope of the Report
- 1.4. Inclusions and Exclusions
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.3. Project Methodology
- 2.4. Forecast Methodology
- 2.5. Robust Quality Control
- 2.6. Key Market Segmentations
- 2.7. Key Considerations
- 2.7.1. Demographics
- 2.7.2. Economic Factors
- 2.7.3. Government Regulations
- 2.7.4. Supply Chain
- 2.7.5. COVID Impact / Related Factors
- 2.7.6. Market Access
- 2.7.7. Healthcare Policies
- 2.7.8. Industry Consolidation
3. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Forecasted Estimates
- 3.2.2. Currency Coverage
- 3.2.2.1. Overview of Major Currencies Affecting the Market
- 3.2.2.2. Impact of Currency Fluctuations on the Industry
- 3.2.3. Foreign Exchange Impact
- 3.2.3.1. Evaluation of Foreign Exchange Rates and Their Impact on Market
- 3.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. EXECUTIVE SUMMARY
5. INTRODUCTION
- 5.1. Chapter Overview
- 5.2. Overview of Cell Culture
- 5.2.1. Classification of Cell Cultures
- 5.2.1.1. Classification based on Origin
- 5.2.1.1.1. Primary Cell Cultures
- 5.2.1.1.2. Secondary Cell Cultures
- 5.2.1.2. Classification based on Growth Properties
- 5.2.1.2.1. Adherent Cell Cultures
- 5.2.1.2.2. Suspension Cell Cultures
- 5.2.1.1. Classification based on Origin
- 5.2.2. Classification of Cell Lines
- 5.2.2.1. Classification based on Lifespan of Cell Line
- 5.2.2.1.1. Finite Cell Lines
- 5.2.2.1.2. Continuous Cell Lines
- 5.2.2.2. Classification based on Type of Cell Line
- 5.2.2.2.1. Recombinant Cell Lines
- 5.2.2.2.2. Hybridoma Cell Lines
- 5.2.2.3. Classification based on Source of Cell Line
- 5.2.2.3.1. Mammalian Cell Lines
- 5.2.2.3.2. Non-Mammalian Cell lines
- 5.2.2.1. Classification based on Lifespan of Cell Line
- 5.2.1. Classification of Cell Cultures
- 5.3. Overview of Cell Line Characterization
- 5.3.1. Cell Line Characterization Methods
- 5.3.1.1. Identity / Stability Testing
- 5.3.1.1.1. Karyotype Analysis
- 5.3.1.1.2. Cytochrome C Oxidase I Barcoding Assay
- 5.3.1.1.3. Cell Morphology Analysis
- 5.3.1.1.4. DNA Analysis
- 5.3.1.2. Sterility / Biosafety Testing
- 5.3.1.2.1. Mycoplasma Contamination Testing
- 5.3.1.2.2. Viral Contamination Testing
- 5.3.1.1. Identity / Stability Testing
- 5.3.1. Cell Line Characterization Methods
- 5.4. Applications of Cell Lines
- 5.5. Key Concerns Associated with Cell Lines
- 5.6. Need for Outsourcing Cell Line Related Operations
- 5.7. Concluding Remarks
6. CELL LINE DEVELOPMENT SERVICE PROVIDERS: MARKET LANDSCAPE
- 6.1. Chapter Overview
- 6.2. Cell Line Development Service Providers: Overall Market Landscape
- 6.2.1. Analysis by Year of Establishment
- 6.2.2. Analysis by Company Size
- 6.2.3. Analysis by Location of Headquarters
- 6.2.4. Analysis by Company Size and Location of Headquarters
- 6.2.5. Analysis by Source of Cell Line / Expression System Offered
- 6.2.6. Analysis by Gene Delivery Method Used
- 6.2.7. Analysis by Type of Transfection
- 6.2.8. Analysis by Availability of Serum-Free / Animal Component Free Culturing Capability
- 6.2.9. Analysis by Type of Cells Offered
- 6.2.10. Analysis by Application of Cell Lines
- 6.2.11. Analysis by Additional Cell Line Related Services Offered
- 6.2.12. Analysis by Type of Cell Banking
- 6.2.13. Analysis by Availability of Integrated Cell Line Characterization Service
7. CELL LINE DEVELOPMENT SERVICE PROVIDERS: COMPANY COMPETITIVENESS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Assumptions / Key Parameters
- 7.3. Methodology
- 7.4. Cell Line Development Service Providers: Company Competitiveness Analysis
- 7.4.1. Cell Line Development Service Providers in North America
- 7.4.2. Cell Line Development Service Providers in Europe
- 7.4.3. Cell Line Development Service Providers in Asia-Pacific
8. COMPANY PROFILES: CELL LINE DEVELOPMENT SERVICE PROVIDERS
- 8.1. Chapter Overview
- 8.2. Detailed Company Profiles of Leading Service Providers
- 8.2.1. ATUM
- 8.2.1.1. Company Overview
- 8.2.1.2. Cell Line Development Service Portfolio
- 8.2.1.3. Recent Developments and Future Outlook
- 8.2.2. Curia
- 8.2.2.1. Company Overview
- 8.2.2.2. Cell Line Development Service Portfolio
- 8.2.2.3. Recent Developments and Future Outlook
- 8.2.3. Fujifilm Diosynth Biotechnologies
- 8.2.3.1. Company Overview
- 8.2.3.2. Service Portfolio for Cell Line Development
- 8.2.3.3. Recent Developments and Future Outlook
- 8.2.4. Lonza
- 8.2.4.1. Company Overview
- 8.2.4.2. Cell Line Development Service Portfolio
- 8.2.4.3. Recent Developments and Future Outlook
- 8.2.5. Syngene International
- 8.2.5.1. Company Overview
- 8.2.5.2. Cell Line Development Service Portfolio
- 8.2.5.3. Recent Developments and Future Outlook
- 8.2.6. WuXi Biologics
- 8.2.6.1. Company Overview
- 8.2.6.2. Cell Line Development Service Portfolio
- 8.2.6.3. Recent Developments and Future Outlook
- 8.2.1. ATUM
- 8.3. Short Profiles of Other Prominent Service Providers
- 8.3.1. Biovian
- 8.3.1.1. Company Overview
- 8.3.1.2. Cell Line Development Service Portfolio
- 8.3.2. Celonic
- 8.3.2.1. Company Overview
- 8.3.2.2. Cell Line Development Service Portfolio
- 8.3.3. ChemPartner
- 8.3.3.1. Company Overview
- 8.3.3.2. Cell Line Development Service Portfolio
- 8.3.4. Creative Biogene
- 8.3.4.1. Company Overview
- 8.3.4.2. Cell Line Development Service Portfolio
- 8.3.5. KBI Biopharma
- 8.3.5.1. Company Overview
- 8.3.5.2. Cell Line Development Service Portfolio
- 8.3.6. Kemp Proteins
- 8.3.6.1. Company Overview
- 8.3.6.2. Cell Line Development Service Portfolio
- 8.3.7. KMD Bioscience
- 8.3.7.1. Company Overview
- 8.3.7.2. Cell Line Development Service Portfolio
- 8.3.8. Mycenax Biotech
- 8.3.8.1. Company Overview
- 8.3.8.2. Cell Line Development Service Portfolio
- 8.3.9. ProBioGen
- 8.3.9.1. Company Overview
- 8.3.9.2. Cell Line Development Service Portfolio
- 8.3.10. Thermo Fisher Scientific
- 8.3.10.1. Company Overview
- 8.3.10.2. Cell Line Development Service Portfolio
- 8.3.1. Biovian
9. CELL LINE CHARACTERIZATION SERVICE PROVIDERS: MARKET LANDSCAPE
- 9.1. Chapter Overview
- 9.2. Cell Line Characterization Service Providers: Overall Market Landscape
- 9.2.1. Cell Line Characterization Service Providers Service Providers (Industry Players)
- 9.2.1.1. Analysis by Year of Establishment
- 9.2.1.2. Analysis by Company Size
- 9.2.1.3. Analysis by Location of Headquarters
- 9.2.1.4. Analysis by Location of Headquarters and Company Size
- 9.2.1.5. Analysis by Cell line Characterized
- 9.2.1.6. Analysis by Type of Cell Line Characterization Service Offered
- 9.2.1.6.1. Analysis by Type of Cell Line Identity / Stability Testing Service Offered
- 9.2.1.6.2. Analysis by Type of Cell Line Sterility / Biosafety Testing Service Offered
- 9.2.2. Cell Line Characterization Service Providers (Non-Industry Players)
- 9.2.2.1. Analysis by Location of Organization
- 9.2.2.2. Analysis by Types of Cell Line Characterized
- 9.2.2.3. Analysis by Type of Cell Line Characterization Service Offered
- 9.2.2.4. Analysis by Genotyping Kit Used
- 9.2.2.5. Analysis by Number of Loci Amplified
- 9.2.1. Cell Line Characterization Service Providers Service Providers (Industry Players)
10. CELL LINE CHARACTERIZATION SERVICE PROVIDERS: COMPANY COMPETITIVENESS ANALYSIS
- 10.1. Chapter Overview
- 10.2. Assumptions / Key Parameters
- 10.3. Methodology
- 10.4. Cell Line Characterization Service Providers: Company Competitiveness Analysis
- 10.4.1. Cell Line Characterization Service Providers in North America
- 10.4.2. Cell Line Characterization Service Providers in Europe
- 10.4.3. Cell Line Characterization Service Providers in Asia-Pacific
11. COMPANY PROFILES: CELL LINE CHARACTERIZATION SERVICE PROVIDERS
- 11.1. Chapter Overview
- 11.2. Detailed Company Profiles of Leading Service Provider
- 11.2.1 Charles River Laboratories
- 11.2.1.1. Company Overview
- 11.2.1.2. Cell Line Characterization Service Portfolio
- 11.2.1.3. Recent Developments and Future Outlook
- 11.2.2. Eurofins BioPharma Product Testing
- 11.2.2.1. Company Overview
- 11.2.2.2. Cell Line Characterization Service Portfolio
- 11.2.2.3. Recent Developments and Future Outlook
- 11.2.3. Livogen Pharmed
- 11.2.3.1. Company Overview
- 11.2.3.2. Cell Line Characterization Service Portfolio
- 11.2.3.3. Recent Developments and Future Outlook
- 11.2.4. Molecular Diagnostic Services
- 11.2.4.1. Company Overview
- 11.2.4.2. Cell Line Characterization Service Portfolio
- 11.2.4.3. Recent Developments and Future Outlook
- 11.2.5. Sartorius
- 11.2.5.1. Company Overview
- 11.2.5.2. Cell Line Characterization Service Portfolio
- 11.2.5.3. Recent Developments and Future Outlook
- 11.2.6. TFBS Bioscience
- 11.2.6.1. Company Overview
- 11.2.6.2. Cell Line Characterization Service Portfolio
- 11.2.6.3. Recent Developments and Future Outlook
- 11.2.1 Charles River Laboratories
- 11.3. Short Profiles of Other Prominent Service Providers
- 11.3.1. ATZ Labs (Subsidiary of Life Technologies)
- 11.3.1.1. Company Overview
- 11.3.1.2. Cell Line Characterization Service Portfolio
- 11.3.2. Avance Biosciences
- 11.3.2.1. Company Overview
- 11.3.2.2. Cell Line Characterization Service Portfolio
- 11.3.3. BioReliance
- 11.3.3.1. Company Overview
- 11.3.3.2. Cell Line Characterization Service Portfolio
- 11.3.4. Clean Cells
- 11.3.4.1. Company Overview
- 11.3.4.2. Cell Line Characterization Service Portfolio
- 11.3.5. hylabs
- 11.3.5.1. Company Overview
- 11.3.5.2. Cell Line Characterization Service Portfolio
- 11.3.6. Samsung Biologics
- 11.3.6.1. Company Overview
- 11.3.6.2. Cell Line Characterization Service Portfolio
- 11.3.7. SGS Life Sciences
- 11.3.7.1. Company Overview
- 11.3.7.2. Cell Line Characterization Service Portfolio
- 11.3.8. Texcell
- 11.3.8.1. Company Overview
- 11.3.8.2. Cell Line Characterization Service Portfolio
- 11.3.9. WuXi Advanced Therapies
- 11.3.9.1. Company Overview
- 11.3.9.2. Cell Line Characterization Service Portfolio
- 11.3.1. ATZ Labs (Subsidiary of Life Technologies)
12. PARTNERSHIPS AND COLLABORATIONS
- 12.1. Chapter Overview
- 12.2. Partnership Models
- 12.3. Cell Line Development and Characterization: Partnerships and Collaborations
- 12.3.1. Analysis by Year of Partnership
- 12.3.2. Analysis by Type of Partnership
- 12.3.3. Analysis by Year and Type of Partnership
- 12.3.4. Analysis by Type of Partner
- 12.3.5. Most Active Players: Analysis by Number of Partnerships
- 12.3.6. Analysis by Geography
- 12.3.6.1. Intracontinental and Intercontinental Deals
- 12.3.6.2. International and Local Deals
13. RECENT EXPANSIONS
- 13.1. Chapter Overview
- 13.2. Cell Line Development and Characterization: List of Recent Expansions
- 13.2.1. Analysis by Year of Expansion
- 13.2.2. Analysis by Type of Expansion
- 13.2.3. Analysis by Year and Type of Expansion
- 13.2.4. Analysis by Location of Headquarters and Company Size
- 13.2.5. Analysis by Location of Expanded Facility
- 13.2.6. Analysis by Type of Expansion and Location of Expanded Facility
- 13.2.7. Analysis by Area of Expanded Facility (sq ft)
- 13.2.8. Analysis by Purpose of Expansion
- 13.2.9. Analysis by Geography
- 13.2.10. Most Active Players: Analysis by Number of Recent Expansions
14. CELL LINE REPOSITORIES
- 14.1. Chapter Overview
- 14.2. American Type Culture Collection (ATCC)
- 14.2.1. Overview
- 14.2.2. Service Portfolio
- 14.3. Coriell Institute for Medical Research
- 14.3.1. Overview
- 14.3.2. Service Portfolio
- 14.4. European Collection of Authenticated Cell Cultures (ECACC)
- 14.4.1. Overview
- 14.4.2. Service Portfolio
- 14.5. Leibniz Institute DSMZ - German Collection of Microorganisms and Cell Cultures
- 14.5.1. Overview
- 14.5.2. Service Portfolio
- 14.6. National Centre for Cell Science (NCCS)
- 14.6.1. Overview
- 14.6.2. Service Portfolio
15. REGULATORY RECOMMENDATIONS AND GUIDELINES
- 15.1. Chapter Overview
- 15.2. Cell Line Authentication: Prominent Regulatory Authorities
- 15.2.1. Role of American Type Culture Collection (ATCC)
- 15.2.2. Role of International Cell Line Authentication Committee (ICLAC)
- 15.2.3. Role of Global Biological Standards Institute (GBSI)
- 15.3. Guidelines Issued by International Regulatory Agencies
- 15.3.1. World Health Organization
- 15.3.2. The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH)
- 15.4. Regulatory Guidelines for Cell Line Characterization in the US
- 15.4.1. Food and Drug Administration
- 15.4.1.1. FDA Points to Consider
- 15.4.1.2. US Pharmacopeial Convention
- 15.4.1.3. US Code for Federal Regulations
- 15.4.1. Food and Drug Administration
- 15.5. Regulatory Guidelines for Cell Line Characterization in Europe
- 15.5.1. European Medicines Agency
- 15.6. Regulatory Guidelines for Cell Line Characterization In Japan
- 15.6.1. Ministry of Health, Labor and Welfare
- 15.7. Concluding Remarks
16. MARKET IMPACT ANALYSIS: DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES
- 16.1. Chapter Overview
- 16.2. Market Drivers
- 16.3. Market Restraints
- 16.4. Market Opportunities
- 16.5. Market Challenges
- 16.6. Conclusion
17. GLOBAL CELL LINE DEVELOPMENT SERVICES MARKET
- 17.1. Chapter Overview
- 17.2. Assumptions and Methodology
- 17.3. Global Cell Line Development Services Market, Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 17.3.1. Scenario Analysis
- 17.3.1.1. Conservative Scenario
- 17.3.1.2. Optimistic Scenario
- 17.3.1. Scenario Analysis
- 17.4. Key Market Segmentations
18. CELL LINE DEVELOPMENT SERVICES MARKET, BY SOURCE OF CELL LINES
- 18.1. Chapter Overview
- 18.2. Key Assumptions and Methodology
- 18.3. Cell Line Development Services Market: Distribution by Source of Cell Lines
- 18.3.1. Cell Line Development Services Market for Mammalian Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 18.3.2. Cell Line Development Services Market for Microbial Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 18.3.3. Cell Line Development Services Market for Insect Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 18.3.4. Cell Line Development Services Market for Other Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 18.4. Data Triangulation and Validation
19. CELL LINE DEVELOPMENT SERVICES MARKET, BY APPLICATION OF CELL LINES
- 19.1. Chapter Overview
- 19.2. Key Assumptions and Methodology
- 19.3. Cell Line Development Services Market: Distribution by Application of Cell Lines
- 19.3.1. Cell Line Development Services Market for Biomanufacturing: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 19.3.2. Cell Line Development Services Market for Research and Development Operations: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 19.4. Data Triangulation and Validation
20. CELL LINE DEVELOPMENT SERVICES MARKET, BY COMPANY SIZE
- 20.1. Chapter Overview
- 20.2. Key Assumptions and Methodology
- 20.3. Cell Line Development Services Market: Distribution by Company Size
- 20.3.1. Cell Line Development Services Market for Large Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 20.3.2. Cell Line Development Services Market for Mid-sized Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 20.3.3. Cell Line Development Services Market for Small Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 20.4. Data Triangulation and Validation
21. CELL LINE DEVELOPMENT SERVICES MARKET, BY KEY GEOGRAPHICAL REGIONS
- 21.1. Chapter Overview
- 21.2. Key Assumptions and Methodology
- 21.3. Cell Line Development Services Market: Distribution by Company Size
- 21.3.1. Cell Line Development Services Market for North America: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 21.3.2. Cell Line Development Services Market for Europe: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 21.3.3. Cell Line Development Services Market for Asia-Pacific and Rest of the World: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 21.4. Data Triangulation and Validation
22. GLOBAL CELL LINE CHARACTERIZATION SERVICES MARKET
- 22.1. Chapter Overview
- 22.2. Assumptions and Methodology
- 22.3. Global Cell Line Characterization Services Market, Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 22.3.1. Scenario Analysis
- 22.3.1.1. Conservative Scenario
- 22.3.1.2. Optimistic Scenario
- 22.3.1. Scenario Analysis
- 22.4. Key Market Segmentations
23. CELL LINE CHARACTERIZATION SERVICES MARKET, BY SOURCE OF CELL LINES
- 23.1. Chapter Overview
- 23.2. Key Assumptions and Methodology
- 23.3. Cell Line Characterization Services Market: Distribution by Source of Cell Lines
- 23.3.1. Cell Line Characterization Services Market for Mammalian Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 23.3.2. Cell Line Characterization Services Market for Microbial Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 23.3.3. Cell Line Characterization Services Market for Insect Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 23.3.4. Cell Line Characterization Services Market for Other Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 23.4. Data Triangulation and Validation
24. CELL LINE CHARACTERIZATION SERVICES MARKET, BY APPLICATION OF CELL LINES
- 24.1. Chapter Overview
- 24.2. Key Assumptions and Methodology
- 24.3. Cell Line Characterization Services Market: Distribution by Application of Cell Lines
- 24.3.1. Cell Line Characterization Services Market for Biomanufacturing: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 24.3.2. Cell Line Characterization Services Market for Research and Development Operations: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 24.4. Data Triangulation and Validation
25. CELL LINE CHARACTERIZATION SERVICES MARKET, BY COMPANY SIZE
- 25.1. Chapter Overview
- 25.2. Key Assumptions and Methodology
- 25.3. Cell Line Characterization Services Market: Distribution by Company Size
- 25.3.1. Cell Line Characterization Services Market for Large Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 25.3.2. Cell Line Characterization Services Market for Mid-sized Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 25.3.3. Cell Line Characterization Services Market for Small Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 25.4. Data Triangulation and Validation
26. CELL LINE CHARACTERIZATION SERVICES MARKET, BY KEY GEOGRAPHICAL REGIONS
- 26.1. Chapter Overview
- 26.2. Key Assumptions and Methodology
- 26.3. Cell Line Characterization Services Market: Distribution by Key Geographical Regions
- 26.3.1. Cell Line Characterization Services Market for North America: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 26.3.2. Cell Line Characterization Services Market for Europe: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 26.3.3. Cell Line Characterization Services Market for Asia-Pacific and Rest of the World: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 26.4. Data Triangulation and Validation
27. CONCLUDING REMARKS
28. EXECUTIVE INSIGHTS
- 28.1. Chapter Overview
- 28.2. Company A
- 28.2.1. Company Snapshot
- 28.2.2. Interview Transcript, Chief Executive Officer
- 28.3. Company B
- 28.3.1. Company Snapshot
- 28.3.2. Interview Transcript, Principal Scientist and Head of R&D
- 28.4. Company C
- 28.4.1. Company Snapshot
- 28.4.2. Interview Transcript, Director of Business Development and Marketing
- 28.5. Company D
- 28.5.1. Company Snapshot
- 28.5.2. Interview Transcript, Founder and President
- 28.6. Company E
- 28.6.1. Company Snapshot
- 28.6.2. Interview Transcript, President
- 28.7. Company F
- 28.7.1. Company Snapshot
- 28.7.2. Interview Transcript, Chief Scientific Officer
- 28.8. Company G
- 28.8.1. Company Snapshot
- 28.8.2. Interview Transcript, Former Vice President BioProcessing
- 28.9. Company H
- 28.9.1. Company Snapshot
- 28.9.2. Interview Transcript, Founder and Managing Director
- 28.10. Company I
- 28.10.1. Company Snapshot
- 28.10.2. Interview Transcript, Client Relations Manager
- 28.11. Company J
- 28.11.1. Company Snapshot
- 28.11.2. Interview Transcript, Former Business Development Manager



















