
|
시장보고서
상품코드
1849803
CAR T 세포치료 시장 : 업계 동향과 세계 예측 - 표적 항원별, 적응증별, 주요 지역별, 의약품 판매 예측, 주요 참여 기업CAR T-Cell Therapy Market: Industry Trends and Global Forecasts - Distribution by Target Antigens, Target Indication, Key Geographies, Sales Forecast of Drugs and Leading Players |
||||||
세계의 CAR T 세포치료 시장 규모는 2035년까지의 예측 기간 중 11.4%의 CAGR로 확대하며, 현재 46억 달러에서 2035년까지 152억 달러로 성장할 것으로 예측됩니다.
시장 세분화에서는 시장 규모와 시장 기회를 다음과 같은 파라미터로 구분하고 있습니다.
적응증
- 급성 림프모구백혈병
- 미만성 대세포 B세포 림프종
- 여포성 림프종
- 대세포 B세포 림프종
- 맨틀세포 림프종
- 다발성골수종
- 기타
표적 항원
- CD19
- BCMA
- CD20
- CD19/CD22
- 기타
주요 지역
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 프랑스
- 이탈리아
- 스페인
- 영국
- 기타 유럽
- 아시아태평양
- 일본
- 인도
- 중국
- 호주
- 한국
- 기타 아시아태평양
- 라틴아메리카
- 중동 및 북아프리카
- 기타 지역
주요 의약품
- 예스카르타
- 김리아
- 아벡마
- 브리앙지
- 칼빅티
- 테카르타스
- 기타
CAR T 세포치료제 시장 : 성장과 동향
암 연구는 제약업계에서 의약품 개발이 가장 활발한 분야 중 하나입니다. 실제로 최근 미국 식품의약국(USFDA)은 다양한 암 치료에 대해 70개 이상의 의약품을 승인했습니다. 기존 치료의 어려움으로 인해 제약 연구자들은 보다 집중적인 항암제 치료법을 적극적으로 모색하고 있습니다. 그 중 CAR T세포치료가 유망 대안으로 떠오르고 있습니다. CAR T세포 치료는 신체의 자연 면역반응을 이용하여 암세포를 찾아내어 파괴하는 치료법입니다. CAR T 세포 치료는 질병의 완전관해를 달성하고 추가 치료의 필요성을 없앨 수 있는 능력을 보여주었습니다. 또한 많은 연구를 통해 이러한 치료법의 효과도 확인되었습니다. CAR T 세포 치료와 관련된 임상시험은 지난 8년 동안 1,000건 가까이 등록되어 많은 연구 노력이 이루어지고 있음을 알 수 있습니다. 현재 230개 이상의 기업이 다양한 암, 비암, 기타 질환의 치료를 위해 CAR T세포 치료제 개발에 참여하고 있습니다.
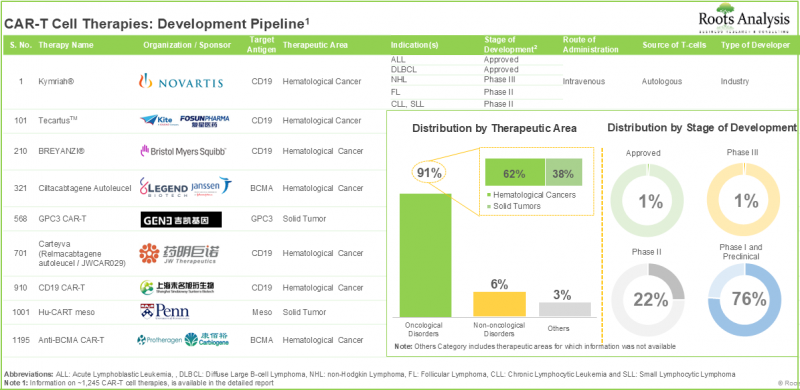
CAR T 세포 치료 시장의 성장은 분자 연구의 기술적 진보, 맞춤형 암 치료에 대한 수요 증가, 임상시험 수 증가, 여러 치료법의 승인에 의해 이루어질 것입니다. 또한 투자자들의 지속적인 자금 지원이 중장기적으로 CAR T세포치료제 개발의 꾸준한 성장을 가속할 것으로 기대됩니다.
CAR T 세포 치료제 시장 주요 인사이트
이 보고서는 CAR T 세포 치료제 시장의 현황을 살펴보고, 업계내 잠재적인 성장 기회를 파악합니다. 이 보고서의 주요 조사 결과는 다음과 같습니다.
- CAR T 세포치료제는 1,240개 이상의 전임상/임상 제품 후보를 보유하고 있으며, 제약 분야에서 가장 활발한 분야 중 하나입니다.
- 다양한 질환을 대상으로 개발중인 치료제 후보물질의 75% 가량이 자가 유래이며, 특히 CD19와 BCMA가 가장 인기 있는 표적 항원으로 부상하고 있습니다.
- CAR construct를 세대별로 개선하기 위해 다양한 유전자 도입 벡터를 사용하여 scFv 영역을 변경하는 등 광범위한 노력이 계속되고 있습니다.
- 다양한 유형의 세포 치료제를 제조하는 데 필요한 역량을 보유하고 있다고 주장하는 기업은 거의 200개에 달하며, 이러한 기업은 제품 개발의 다양한 단계에 걸쳐 광범위한 서비스를 제공합니다.
- 지난 8년간 CAR T세포 치료와 관련된 임상시험은 다양한 지역에서 970건 이상 등록되었습니다.
- 현재 유명 대학의 375명 이상의 과학자들이 CAR T 세포 치료의 임상 개발에 참여하고 있으며, 이들 KOL은 주로 미국과 중국에 기반을 두고 있습니다.
- 최근 국제 이해관계자와 현지 이해관계자가 모두 참여하는 제휴가 증가하고 있으며, 이는 이 분야에 대한 관심이 높아지고 있다는 것을 증명하고 있습니다.
- 암 면역치료라는 미래 사업 기회를 발견한 여러 투자자들이 370여 건에 걸쳐 320억 달러 이상을 투자하고 있습니다.
- CAR T 세포치료 관련 특허는 11,900건 이상 출원되어 이 분야에서 탄생한 지적 재산을 보호하고 있습니다.
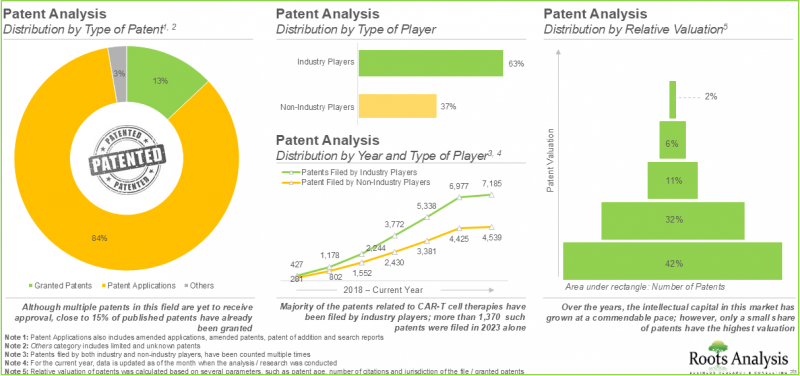
- 이러한 치료제를 효율적으로 홍보하고 CAR T세포치료제 시장에서의 지위를 유지하기 위해 의약품 개발 기업은 적극적으로 다양한 홍보 전략을 모색하고 있습니다.
- CAR T 세포치료제 시장은 암 발병률 증가, 기술 개발, 진행 중인 승인 등을 고려할 때 향후 꾸준한 성장세를 보일 것으로 예측됩니다.
- 개발 파이프라인에 대한 관심 증가와 유망 임상 결과에 힘입어 향후 10년간 연평균 11.4% 이상 성장할 것으로 예측됩니다.
CAR T 세포 치료제 시장 주요 부문
예측 기간 중 다발성골수종은 CAR T 세포 치료제 시장을 독점할 가능성이 높습니다.
적응증별로 시장은 급성 림프모구백혈병, 미만성 대세포 B세포 림프종, 여포성 림프종, 대세포 B세포 림프종, 맨틀세포 림프종, 다발성골수종, 기타로 구분됩니다. 대세포 B세포 림프종 치료를 위한 CAR T 치료제 시장은 예측 기간 중 연간 14%의 성장률로 성장할 것으로 예상된다는 점은 주목할 만합니다.
예측 기간 중 CD19 표적 치료제가 CAR T 세포치료제 시장에서 가장 큰 점유율을 차지할 것으로 예측됩니다.
표적 항원별로 시장은 CD19, BCMA, CD20, CD19/CD22, 기타로 구분됩니다. 주목할 만한 점은 CD19 부문이 향후 수년간 전체 시장의 65% 이상을 차지할 가능성이 높다는 점입니다. 이는 Kymriah(R)(tisagenlecleucel), Yescarta(R)(axicabtagene ciloleucel) 등 CD19를 표적으로 하는 치료제의 성공에 기인합니다.
북미가 가장 큰 시장 점유율을 차지합니다.
주요 지역별로 시장은 북미, 유럽, 아시아태평양, 라틴아메리카, 중동 및 북아프리카, 기타 지역으로 구분됩니다. 주목할 만한 점은 아시아태평양 시장이 향후 높은 CAGR로 성장할 것으로 예상된다는 점입니다.
CAR T 세포치료제 시장 진출기업 사례
- Autolus
- Bluebird Bio
- Bristol Myers Squibb
- Carsgen Therapeutics
- Cellectis, Gilead Sciences
- Innovative Cellular Therapeutics
- Noile-Immune Biotech
- Novartis
- Shanghai GeneChem
- Sinobioway Cell Therapy
- Takara Bio
- Wellington Zhaotai Therapies
1차 조사 개요
본 조사에서 제시된 의견과 인사이트는 여러 이해관계자와의 논의를 통해 영향을 받았습니다. 본 조사 보고서에는 다음과 같은 업계 이해관계자들과의 인터뷰 내용을 상세하게 수록하고 있습니다.
- A사 대표이사
- B사 CEO
- C사 최고운영책임자
- D사 과학 담당 부사장
- E사 면역종양학 담당 부사장
- F사 와이즈만 바이오매뉴팩처링 전략 및 사업개발 담당 경쟁정보 매니저 및 사업개발 매니저
- G사 의대 교수 겸 종양내과 과장
- H사 의학 조교수
CAR T 세포치료제 시장 조사 대상
- 시장 예측 및 기회 분석 : 이 보고서는 CAR T 세포치료제 시장을 상세하게 분석하고,(A) 표적 항원,(B) 적응증,(C) 주요 지역 분석,(D) 의약품 매출 예측,(E) 주요 기업 등 주요 시장 부문에 초점을 맞추었습니다.
- 시장 영향 분석 : 시장 성장에 영향을 미치는(A) 촉진요인,(B) 억제요인,(C) 시장 성장 촉진요인,(D) 과제 등 다양한 요인을 분석했습니다.
- 시장 상황: A) 개발사 유형, B) 개발 단계, C) 치료 영역, D) 주요 적응증, E) 주요 표적 항원, F) T세포 공급원, G) 투여 경로, H) 투여 횟수, I) 환자 부문, J) 치료법 유형, K) 설립 년도, L) 기업 규모, M) 본사 소재지 등의 다양한 매개변수를 고려하여 CAR T 세포 치료제의 현재 시장 상황을 종합적으로 평가합니다.
- 임상시험 분석 :(A) 임상시험 등록 연도,(B) 등록 환자 수,(C) 임상시험 모집 현황,(D) 임상시험 단계,(E) 대상 환자군,(F) 스폰서/공동연구자 유형,(G) 가장 활동적인 기업,(H) 임상시험의 지역적 분포 등의 매개변수를 기준으로 완료, 진행 중, 계획 중인 임상시험을 상세하게 분석. 계획 중인 임상시험을 상세하게 분석.
- 주요 인사이트 혈액 악성 종양 및 고형암과 관련된 일반적인 표적 항원에 중점을 둔 경쟁 분석을 특징으로 하는 연구에서 얻은 주요 고려 사항 분석. 또한(A) CAR의 세대,(B) 결합 도메인 유형,(C) 사용된 바이러스 유형,(D) 사용된 유전자 도입 방법 유형,(E) 사용된 공동 자극 도메인 유형에 따라 임상 단계의 CAR T 세포 치료의 CAR 구조 분석을 포함합니다.
- KOL: Key Opinion Leaders: 이 분야의 핵심 오피니언 리더(KOL)에 대한 상세한 분석에는 CAR T 치료와 관련된 임상시험을 감독하는 다양한 책임연구자들에 대한 평가가 포함됩니다. 이들 임상시험책임의사는 CAR T세포치료제 연구개발에 적극적으로 참여하고 있으며, KOL로 인정받고 있습니다. 또한 본 장에서는 제3자 평가별 평가와 함께 독자적인 스코어링 시스템을 활용하여 KOL의 전문지식을 비교 분석했습니다.
- 기업 개요:(A) 기업 개요,(B) CAR T 세포치료에 특화된 제품 포트폴리오,(C) 기술 포트폴리오(있는 경우),(D) CAR T 세포치료 관련 최근 동향, 기업의 제조 능력에 초점을 맞춘 CAR T 세포치료 시장 주요 기업의 상세 프로파일. 또한 이들 기업에 대한 전략적/벤처캐피털 투자에 대한 자세한 내용도 포함되어 있습니다.
- 파트너십 및 협업: A) 제휴 연도, B) 제휴 유형, C) 제휴 대상 유형, D) 지역 분포 등 몇 가지 관련 매개 변수를 기반으로 이 영역에서 구축된 파트너십을 분석합니다.
- 자금 조달 및 투자 분석 :(A) 자금 조달 연도,(B) 자금 조달 유형,(C) 투자 금액,(D) 지역 분포 등 몇 가지 관련 매개 변수를 기반으로 이 영역에서 보고된 자금 조달 및 투자 분석.
- 특허 분석 :(A) 특허 유형,(B) 공개 연도,(C) 지역 분포,(D) 특허 협력 분류(CPC) 기호,(E) 새로운 중점 분야,(F) 출원인 유형,(G) 주요 기업(특허 출원/허여 건수 기준),(H) 특허 평가,(I) 특허 벤치마킹을 기준으로 CAR T 세포 치료 관련 출원 또는 부여된 11,900건 이상의 특허를 상세하게 분석.
- 사례 연구 세포치료제 제조에 초점을 맞춘 사례 연구로, 과제를 강조하고 이 분야와 관련된 위탁 서비스 프로바이더와 자체 제조업체의 종합적인 리스트을 제공합니다.
- 비용 가격 분석 : 세포치료제 가격 책정에 기여하는 다양한 요인을 종합적으로 평가. 가까운 미래에 출시될 것으로 예상되는 CAR T 세포 치료제의 가격을 결정할 때 제약사가 고려할 수 있는 다양한 모델과 전략도 포함되어 있습니다.
목차
제1장 서문
제2장 조사 방법
제3장 경제적 및 기타 프로젝트 특유 고려 사항
제4장 개요
제5장 서론
- 챕터 개요
- T 세포 면역요법의 개요
- 역사적 진화
- T 세포 면역요법의 개발에서의 주요 고려사항
- T세포의 방향 전환에 이용되는 전략
- 개변 T세포의 제조
- 키메라 항원 수용체 T세포(CAR T) 요법
- 개발의 역사
- CAR 구조
- CAR T세포의 개발
- 유니버설 CAR T세포
- 투여 경로
- CAR T 세포치료에 수반하는 과제
- 결론
제6장 CAR T 세포치료 : 시장 구도
- 챕터 개요
- CAR T 세포치료 : 시장 구도
- CAR T 세포치료 : 개발 기업의 전체 상황
제7장 주요 인사이트
- 챕터 개요
- CAR T 세포치료 : 인기 표적 항원
- 조혈 악성 종양 표적
- 고형 종양의 일반적인 표적
- CAR T요법 : CAR 구조 해석
제8장 임상시험 분석
- 챕터 개요
- 범위와 조사 방법
- CAR T 세포치료 : 임상시험 분석
제9장 주요 오피니언 리더
- 챕터 개요
- 전제와 주요 파라미터
- 조사 방법
- CAR T 세포치료 : 주요 오피니언 리더
제10장 기업 개요
- 챕터 개요
- Autolus
- Bluebird Bio
- Bristol Myers Squibb
- CARsgen Therapeutics
- Cellectis
- Gilead Sciences
- Innovative Cellular Therapeutics
- Noile-Immune Biotech
- Novartis
- Sinobioway Cell Therapy
- Takara Bio
- Wellington Zhaotai Therapies
제11장 파트너십과 협업
- 챕터 개요
- 파트너십 모델
- CAR T 세포치료 : 파트너십과 협업
제12장 자금조달과 투자 분석
- 챕터 개요
- 자금조달의 유형
- CAR T 세포치료 : 자금조달과 투자 분석
제13장 특허 분석
- 챕터 개요
- 범위와 조사 방법
- CAR T 세포치료 : 특허 분석
- 특허 벤치마킹 분석
- 특허 평가
- 인용수 많은 특허
제14장 사례 연구 : 세포치료 제조
- 챕터 개요
- 세포치료 제조의 개요
- 세포치료 제조 모델
- 집중형 제조 모델
- 분산형 제조 모델
- 세포치료 제조 프로세스의 확장성
- 세포치료 제조업체의 유형
- 세포치료의 제조에 관련된 주요 과제
- 세포 치료제 제조에서의 주요 고려사항
- 세포치료 제조 프로세스의 자동화
- 세포치료 제조 공급망
- 자사 제조 능력을 가진 기업과 위탁 제조 기업의 비교
- 규제 상황
- 향후 전망
제15장 원가분석
- 챕터 개요
- 세포·유전자 치료의 고가격화에 기여하는 요인
- T 세포 면역요법의 가격 모델
- T 세포 면역요법의 상환에 관한 고려사항
제16장 시장 영향 분석 : 촉진요인, 제약 요인, 기회, 과제
제17장 세계의 CAR T 세포치료 시장
제18장 CAR T 세포치료 시장(표적 항원별)
제19장 CAR T 세포치료 시장(적응증별)
제20장 CAR T 세포치료 시장(주요 지역별)
제21장 CAR T요법 시장, 의약품 판매 예측
- 챕터 개요
- 주요 전제와 조사 방법
- 시판되고 있는 CAR T 세포치료 : 판매 예측
- 임상 CAR T 세포치료 : 판매 예측
- 데이터 삼각측량과 검증
제22장 CAR T 세포치료 시장 : 주요 기업의 판매 예측
- 챕터 개요
- 주요 전제와 조사 방법
- CAR T 세포치료 : 주요 참여 기업
- Gilead Sciences 판매 예측
- Bristol Myers Squibb 판매 예측
- Novartis 판매 예측
- Janssen 판매 예측
- JW Therapeutics 판매 예측
- 데이터 삼각측량과 검증
제23장 프로모션 분석
- 챕터 개요
- 프로모션 캠페인에 사용되는 채널
- 제품 웹사이트 분석의 요약
- 환자 지원 서비스와 정보 다운로드의 요약
- Kymriah : 프로모션 분석
- Yescarta : 프로모션 분석
- Tecartus : 프로모션 분석
- Breyanzi : 프로모션 분석
- Abecma : 프로모션 분석
- Carvykti : 프로모션 분석
제24장 이그제큐티브 인사이트
제25장 결론
제26장 부록 I : 표 데이터
제27장 부록 II : 기업 및 조직 리스트
제28장 부록 III : 자금 및 투자 리스트
KSA 25.11.05CAR T-Cell Therapy Market: Overview
As per Roots Analysis, the global CAR T-cell therapy market is estimated to grow from USD 4.6 billion in the current year to USD 15.2 billion by 2035, at a CAGR of 11.4% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Target Indication
- Acute Lymphoblastic Leukemia
- Diffuse Large B-cell Lymphoma
- Follicular Lymphoma
- Large B-cell Lymphoma
- Mantle Cell Lymphoma
- Multiple Myeloma
- Others
Target Antigen
- CD19
- BCMA
- CD20
- CD19 / CD22
- Others
Key Geographical Regions
- North America
- US
- Canada
- Europe
- Germany
- France
- Italy
- Spain
- UK
- Rest of Europe
- Asia-Pacific
- Japan
- India
- China
- Australia
- South Korea
- Rest of Asia Pacific
- Latin America
- Middle East and North Africa
- Rest of the World
Key Drugs
- Yescarta
- Kymriah
- Abecma
- Breyanzi
- Carvykti
- Tecartus
- Others
CAR T-Cell Therapy Market: Growth and Trends
Cancer research is one of the most active segments for drug development within the pharmaceutical sector. In fact, in recent years, the USFDA has authorized over 70 medications for various cancer treatments. Due to the difficulties linked with traditional treatments, pharmaceutical researchers are actively exploring more focused anti-cancer therapies. Among these, CAR T-cell therapies have appeared as a promising alternative. CAR T-cell treatment utilizes the body's natural immune response to identify and destroy cancer cells. CAR-T cell therapies have shown the ability to achieve total disease remission, eliminating the necessity for further treatment. Further, numerous studies have also confirmed the effectiveness of these therapy options. It is important to note that nearly 1,000 clinical trials associated with CAR-T cell therapies have been registered over the last eight years, showing significant research efforts. Currently, more than 230 firms are involved in development of CAR T-cell therapies for the treatment of different oncological, non-oncological and other disorders.
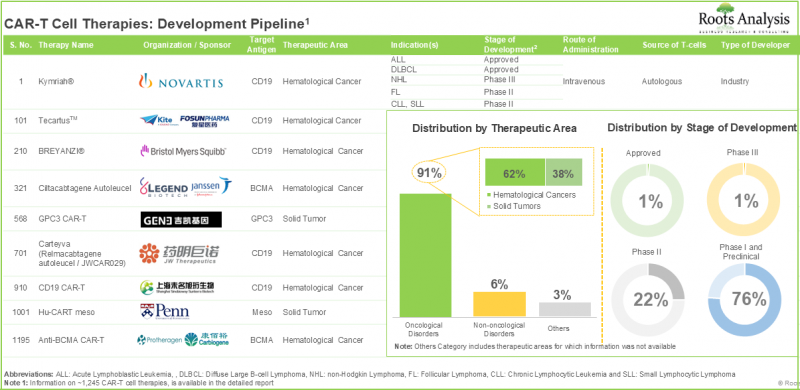
The CAR-T cell therapy market growth is driven by technological advancements in molecular research, the increased demand for personalized cancer treatment and increase in the number of clinical trials and multiple therapy approvals. Further, continuous financial support from investors is expected to propel the steady growth for the development of CAR-T cell therapy in the mid-to-long-term.
CAR T-Cell Therapy Market: Key Insights
The report delves into the current state of the CAR T-cell therapy market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- CAR-T cell therapies, with over 1,240 preclinical / clinical product candidates, represent one of the most active segments in the pharmaceutical domain.
- Close to 75% of the therapy candidates, which are being developed to target a range of disease indications, are autologous in nature; notably, CD19 and BCMA have emerged as the most popular target antigens.
- Extensive efforts are underway to improve CAR constructs across successive generations, involving alterations in the scFv region via using different types of gene delivery vectors.
- Close to 200 players claim to have the required capabilities to manufacture different types of cell therapies; such firms also offer a wide range of services across different stages of product development.
- In the last eight years, over 970 clinical trials have been registered across different geographies for CAR-T cell therapies; extensive efforts are underway to improve successive generations of these therapies.
- 375+ scientists from renowned universities are presently involved in the clinical development of CAR-T cell therapies; these KOLs are primarily based in the US and China.
- The rise in partnerships, in the recent past, involving both international and indigenous stakeholders, validate the growing interest in this domain.
- Several investors, having realized the opportunity within this upcoming segment of cancer immunotherapy, have invested more than USD 32 billion, across 370+ instances.
- More than 11,900 patents related to CAR-T cell therapies have been filed / granted to protect the intellectual property generated within this field.
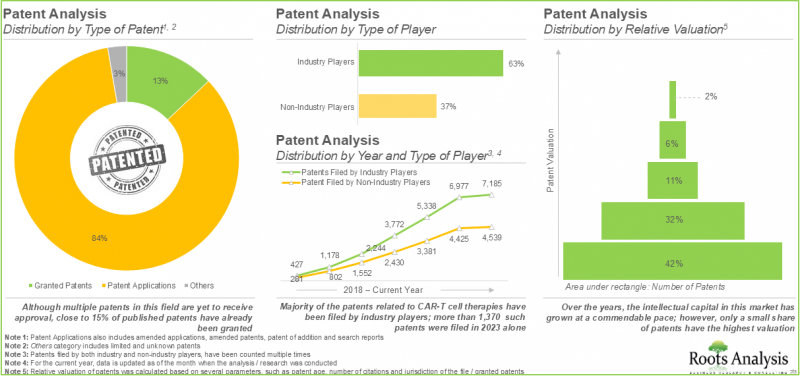
- In order to efficiently promote these therapies and sustain their position in the CAR-T cell therapies market, drug developers are actively exploring diverse promotional strategies.
- Considering the growing prevalence of cancer, technological developments and ongoing approvals, the market for CAR-T cell therapies is poised to witness steady growth in the foreseen future.
- With a growing focus on the development pipeline and encouraging clinical results, the market is expected to witness an annualized growth rate of over 11.4%, during the next decade.
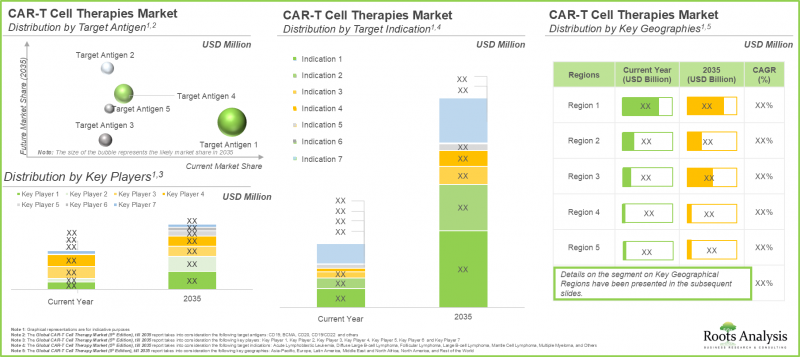
CAR T-Cell Therapy Market: Key Segments
Multiple Myeloma is Likely to Dominate the CAR T-Cell Therapy Market During the Forecast Period
Based on target indication, the market is segmented into acute lymphoblastic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, large B-cell lymphoma, mantle cell lymphoma, multiple myeloma and others. It is worth highlighting that the market for CAR-T therapies designed to treat large B-cell lymphoma is projected to grow at an annual growth rate of 14%, during the forecast period.
CD19 Targeting Therapies is Expected to Capture Largest Share in the CAR T-Cell Therapy Market During the Forecast Period
Based on target antigen, the market is segmented into CD19, BCMA, CD20, CD19 / CD22 and others. It is worth highlighting that the segment for CD19 is likely to capture more than 65% of the overall market in the coming years. This can be attributed to the success of CD19 targeting therapies, such as Kymriah(R) (tisagenlecleucel) and Yescarta(R) (axicabtagene ciloleucel).
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific, Latin America, Middle East and North Africa, and Rest of the World. It is worth highlighting that over the years, the market in Asia-Pacific is expected to grow at a higher CAGR.
Example Players in the CAR T-Cell Therapy Market
- Autolus
- Bluebird Bio
- Bristol Myers Squibb
- Carsgen Therapeutics
- Cellectis, Gilead Sciences
- Innovative Cellular Therapeutics
- Noile-Immune Biotech
- Novartis
- Shanghai GeneChem
- Sinobioway Cell Therapy
- Takara Bio
- Wellington Zhaotai Therapies
Primary Research Overview
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:
- Chief Executive Officer, Company A
- Chief Executive Officer, Company B
- Chief Operating Officer, Company C
- Vice President, Scientific Affairs, Company D
- Vice President, Immuno-Oncology, Company E
- Manager of Business Development, Waisman Biomanufacturing Competitive Intelligence Manager, Strategy & Business Development, Company F
- Professor of Medicine and Director, Department of Oncology, Company G
- Assistant Professor of Medicine, Company H
CAR T-Cell Therapy Market: Research Coverage
- Market Forecast and Opportunity Analysis: The report features an in-depth analysis of the CAR T-cell therapy market, focusing on key market segments, including [A] target antigens, [B] target indication, [C] key geographical regions, [D] sales forecast of drugs and [E] leading players.
- Market Impact Analysis: The report analyzes various factors such as [A] drivers, [B] restraints, [C] opportunities, and [D] challenges affecting market growth.
- Market Landscape: A comprehensive evaluation of the current market landscape of CAR-T cell therapies, considering various parameters, such as [A] type of developer, [B] phase of development, [C] therapeutic area, [D] key target indication, [E] key target antigen, [F] source of T-cells, [G] route of administration, [H] dose frequency, [I] patient segment, [J] type of therapy, [K] year of establishment, [L] company size and [M] location of headquarters.
- Clinical Trial Analysis: A detailed analysis of clinical trials, completed, ongoing, and planned clinical studies based on parameters, such as [A] trial registration year, [B] enrolled patient population, [C] trial recruitment status, [D] trial phase, [E] target patient segment, [F] type of sponsor / collaborator, [G] most active players and [H] regional distribution of trials.
- Key Insights: An analysis of key insights derived from the study featuring a competitive analysis, emphasizing the prevalent target antigens associated with hematological malignancies and solid tumors. Additionally, it includes CAR construct analysis of clinical-stage CAR T cell therapies based on the [A] generation of CAR, [B] type of binding domain, [C] type of virus used, [D] type of gene transfer method used, and [E] type of co-stimulatory domain used.
- Key Opinion Leaders: An in-depth analysis of key opinion leaders (KOLs) within this field includes an evaluation of various principal investigators overseeing clinical trials associated with CAR-T therapies. These investigators are recognized as KOLs due to their active engagement in the research and development of CAR T cell therapies. Additionally, the chapter provides a comparative analysis of the KOLs' expertise, utilizing a proprietary scoring system alongside assessments from third-party evaluations.
- Company Profiles: In-depth profiles of key players in CAR T-cell therapy market, focusing on [A] company overviews, [B] product portfolio specific to CAR-T therapies, [C] technology portfolio (if available), [D] recent developments related to CAR-T cell therapies and manufacturing capabilities of the companies. In addition, it also includes details of the strategic / venture capital investments made in these companies.
- Partnerships and Collaborations: An analysis of partnerships established in this domain, based on several relevant parameters, such as the [A] year of partnership, [B] type of partnership, [C] type of partner, and [D] geographical distribution.
- Funding and Investment Analysis: An analysis of funding and investment reported in this domain, based on several relevant parameters, such as the [A] year of funding, [B] type of funding, [C] amount invested, and [D] geographical distribution.
- Patent Analysis: Detailed analysis of over 11,900 patents filed or granted related to CAR T-cell therapies based on [A] type of patent, [B] publication year, [C] geographical distribution, [D] Cooperative Patent Classification (CPC) symbols, [E] emerging focus areas, [F] type of applicant, [G] leading players (on the basis of number of patents filed / granted), [H] patent valuation and [I] patent benchmarking.
- Case Study: A case study focused on the production of cell therapy products, emphasizing the challenges and providing a comprehensive list of contract service providers and in-house manufacturers involved in this domain.
- Cost Price Analysis: A comprehensive evaluation of the various factors that contribute to the pricing of cell-based therapies. It includes different models and strategies that a pharmaceutical company might consider when determining the price of a CAR-T cell therapy expected to be launched in the near future.
Key Questions Answered in this Report
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What kind of partnership models are commonly adopted by industry stakeholders?
- How many clinical trials are running for CAR-T therapies?
- What are the factors that are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
Reasons to Buy this Report
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
Additional Benefits
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Market Share Insights
- 1.3. Key Market Insights
- 1.4. Report Coverage
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.3. Project Methodology
- 2.4. Forecast Methodology
- 2.5. Robust Quality Control
- 2.6. Key Market Segmentations
- 2.7. Key Considerations
- 2.7.1. Demographics
- 2.7.2. Economic Factors
- 2.7.3. Government Regulations
- 2.7.4. Supply Chain
- 2.7.5. COVID Impact / Related Factors
- 2.7.6. Market Access
- 2.7.7. Healthcare Policies
- 2.7.8. Industry Consolidation
3. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Forecasted Estimates
- 3.2.2. Currency Coverage
- 3.2.2.1. Overview of Major Currencies Affecting the Market
- 3.2.2.2. Impact of Currency Fluctuations on the Industry
- 3.2.3. Foreign Exchange Impact
- 3.2.3.1. Evaluation of Foreign Exchange Rates and Their Impact on Market
- 3.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. EXECUTIVE SUMMARY
5. INTRODUCTION
- 5.1. Chapter Overview
- 5.2. Overview of T-cell Immunotherapies
- 5.2.1. Historical Evolution
- 5.2.2. Key Considerations for Developing T-cell Immunotherapies
- 5.2.3. Strategies Employed for the Redirection of T-cells
- 5.2.4. Manufacturing of Engineered T-cells
- 5.3. Chimeric Antigen Receptor T-cell (CAR-T) Therapy
- 5.3.1. Development History
- 5.3.2. Structure of CAR
- 5.3.2.1. Ectodomain
- 5.3.2.2. Transmembrane (TM) Domain
- 5.3.2.3. Endodomain
- 5.3.3. Development of CAR-T Cells
- 5.3.4. Universal CAR-T Cells
- 5.3.5. Route of Administration
- 5.3.6. Challenges Associated with CAR-T Cell Therapies
- 5.3.6.1. Competitive Risks
- 5.3.6.2. Clinical Risks
- 5.3.6.3. Regulatory Risks
- 5.3.6.4. Commercial Risks
- 5.4. Concluding Remarks
6. CAR-T CELL THERAPIES: MARKET LANDSCAPE
- 6.1. Chapter Overview
- 6.2. CAR-T Cell Therapies: Market Landscape
- 6.2.2. Analysis by Stage of Development
- 6.2.3. Analysis by Type of Therapy
- 6.2.4. Analysis by Target Antigen
- 6.2.5. Analysis by Target Indication
- 6.2.6. Analysis by Therapeutic Area
- 6.2.7. Analysis by Stage of Development and Therapeutic Area
- 6.2.8. Analysis by Source of T-cells
- 6.2.9. Analysis by Stage of Development and Source of T-cells
- 6.2.10. Analysis by Route of Administration
- 6.2.11. Analysis by Dosage Regimen
- 6.2.12. Analysis by Target Patient Population
- 6.2.13. Most Active Industry Players: Analysis by Number of Therapies
- 6.2.14. Most Active Non-Industry Players: Analysis by Number of Therapies
- 6.3. CAR-T Cell Therapies: Overall Developer Landscape
- 6.3.1. Analysis by Year of Establishment
- 6.3.2. Analysis by Company Size
- 6.3.3. Analysis by Location of Headquarters
7. KEY INSIGHTS
- 7.1. Chapter Overview
- 7.2. CAR-T Cell Therapies: Popular Target Antigens
- 7.2.1. Popular Targets for Hematological Malignancies
- 7.2.2. Popular Targets for Solid Tumors
- 7.3. CAR-T Therapies: CAR Construct Analysis
- 7.3.1. Analysis by Generation of CAR
- 7.3.2. Analysis by Type of scFv Antibody Used
- 7.3.3. Analysis by Type of Virus Used
- 7.3.4. Analysis by Type of Gene Transfer Method Used
- 7.3.5. Analysis by Type of Co-Stimulatory Domain
8. CLINICAL TRIAL ANALYSIS
- 8.1. Chapter Overview
- 8.2. Scope and Methodology
- 8.3. CAR-T Cell Therapies: Clinical Trial Analysis
- 8.3.1. Analysis by Trial Registration Year
- 8.3.2. Analysis of Enrolled Patient Population by Trial Registration Year
- 8.3.3. Analysis by Trial Phase
- 8.3.4. Analysis of Enrolled Patient Population by Trial Phase
- 8.3.5. Analysis by Trial Registration Year and Trial Phase
- 8.3.6. Analysis by Trial Status
- 8.3.7. Analysis by Patient Gender
- 8.3.8. Analysis by Therapeutic Area
- 8.3.9. Analysis by Study Design
- 8.3.10. Most Active Industry Players: Analysis by Number of Registered Trials
- 8.3.11. Most Active Non-Industry Players: Analysis by Number of Registered Trials
- 8.3.13. Analysis by Geography
9. KEY OPINION LEADERS
- 9.1. Chapter Overview
- 9.2. Assumptions and Key Parameters
- 9.3. Methodology
- 9.4. CAR-T Cell Therapies: Key Opinion Leaders
- 9.4.1. Analysis by Type of Organization
- 9.4.2. Analysis by Affiliated Organization
- 9.4.3. Analysis by Geographical Location of KOLs
- 9.4.4. KOL Activeness versus KOL Strength
- 9.4.5. Most Prominent KOLs: Analysis by RA score
- 9.4.6. Most Prominent KOLs: Comparison of RA Score and Third-Party Score
10. COMPANY PROFILES
- 10.1. Chapter Overview
- 10.2. Autolus
- 10.3. Bluebird Bio
- 10.4. Bristol Myers Squibb
- 10.5. CARsgen Therapeutics
- 10.6. Cellectis
- 10.7. Gilead Sciences
- 10.8. Innovative Cellular Therapeutics
- 10.9. Noile-Immune Biotech
- 10.10. Novartis
- 10.11. Sinobioway Cell Therapy
- 10.12. Takara Bio
- 10.13. Wellington Zhaotai Therapies
11. PARTNERSHIPS AND COLLABORATIONS
- 11.1. Chapter Overview
- 11.2. Partnership Models
- 11.3. CAR-T Cell Therapies: Partnerships and Collaborations
- 11.3.1. Analysis by Year of Partnership
- 11.3.2. Analysis by Type of Partnership
- 11.3.3. Analysis by Year and Type of Partnership
- 11.3.4. Analysis by Type of Partner
- 11.3.5. Most Popular Therapies: Analysis by Number of Partnerships
- 11.3.6. Most Active Industry Players: Analysis by Number of Partnerships
- 11.3.7. Most Active Non-Industry Players: Analysis by Number of Partnerships
- 11.3.8. Analysis by Geography
- 11.3.8.1. Intercontinental and Intracontinental Agreements
- 11.3.8.2. International and Local Agreements
12. FUNDING AND INVESTMENTS ANALYSIS
- 12.1. Chapter Overview
- 12.2. Types of Funding
- 12.3. CAR-T Cell Therapies: Funding and Investment Analysis
- 12.3.1. Analysis by Year of Funding
- 12.3.2. Analysis of Amount Invested
- 12.3.3. Analysis by Type of Funding
- 12.3.4. Analysis by Amount Invested and Type of Funding
- 12.3.5. Analysis by Amount Invested by year and Type of Funding Analysis by Type of Investor
- 12.3.6. Analysis by Geography
- 12.3.7. Most Active Players
- 12.3.8. Leading Investors: Analysis by Number of Funding Instances
13. PATENT ANALYSIS
- 13.1. Chapter Overview
- 13.2. Scope and Methodology
- 13.3. CAR-T Cell Therapies: Patent Analysis
- 13.3.1. Analysis by Patent Publication Year
- 13.3.2. Analysis by Patent Application Year
- 13.3.3. Analysis of Granted Patents and Patent Applications by Publication Year
- 13.3.4. Analysis by Patent Jurisdiction
- 13.3.5. Analysis by CPC Symbols
- 13.3.6. Analysis by Type of Applicant
- 13.3.7. Leading Industry Players: Analysis by Number of Patents
- 13.3.8. Leading Non-Industry Players: Analysis by Number of Patents
- 13.3.9. Leading Patent Assignees: Analysis by Number of Patents
- 13.4. Patent Benchmarking Analysis
- 13.4.1. Analysis by Patent Characteristics
- 13.5. Patent Valuation
- 13.6. Leading Patents by Number Of Citations
14. CASE STUDY: CELL THERAPY MANUFACTURING
- 14.1. Chapter Overview
- 14.2. Overview of Cell Therapy Manufacturing
- 14.3. Cell Therapy Manufacturing Models
- 14.3.1. Centralized Manufacturing Model
- 14.3.2. Decentralized Manufacturing Model
- 14.4. Scalability of Cell Therapy Manufacturing Processes
- 14.4.1. Scale-Up
- 14.4.2. Scale-Out
- 14.5. Types of Cell Therapy Manufacturers
- 14.6. Key Challenges Related to Manufacturing of Cell Therapies
- 14.7. Key Considerations for Cell Therapy Manufacturing
- 14.7.1. Characterization
- 14.7.2. Cost of Goods
- 14.8. Automation of Cell Therapy Manufacturing Process
- 14.9. Cell Therapy Manufacturing Supply Chain
- 14.10. Comparison of Players Having In-house Capabilities and Contract Manufacturers
- 14.11. Regulatory Landscape
- 14.12. Future Perspectives
15. COST PRICE ANALYSIS
- 15.1. Chapter Overview
- 15.2. Factors Contributing to the High Price of Cell / Gene Therapies
- 15.3. Pricing Models for T-cell Immunotherapies
- 15.3.1. Based on Associated Costs
- 15.3.2. Based on Availability of Competing Products
- 15.3.3. Based on Patient Population
- 15.3.4. Based on Opinions of Industry Experts
- 15.4. Reimbursement related Considerations for T-cell Immunotherapies
- 15.4.1. Case Study: The National Institute for Health and Care Excellence (NICE) Appraisal of CAR-T Therapies
16. MARKET IMPACT ANALYSIS: DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES
- 16.1. Chapter Overview
- 16.2. Market Drivers
- 16.3. Market Restraints
- 16.4. Market Opportunities
- 16.5. Market Challenges
- 16.6. Conclusion
17. GLOBAL CAR-T CELL THERAPY MARKET
- 17.1. Chapter Overview
- 17.2. Key Assumptions and Methodology
- 17.3. Global CAR-T Cell Therapy Market, Historical Trends (since 2018) and Future Estimates (till 2035)
- 17.4. Scenario Analysis
- 17.4.1. Conservative Scenario
- 17.4.2. Optimistic Scenario
- 17.5. Key Market Segmentations
18. CAR-T CELL THERAPY MARKET, BY TARGET ANTIGEN
- 18.1. Chapter Overview
- 18.2. Key Assumptions and Methodology
- 18.3. CAR-T Cell Therapies Market: Distribution by Type Target Antigen, Current Year and 2035
- 18.3.1. CART-T Cell Therapies Market for CD19: Forecasted Estimates (till 2035)
- 18.3.2. CART-T Cell Therapies Market FOR BCMA: Forecasted Estimates (till 2035)
- 18.3.3. CART-T Cell Therapies Market FOR CD20: Forecasted Estimates (till 2035)
- 18.3.4. CART-T Cell Therapies Market for CD19 / CD22: Forecasted Estimates (2025-2035)
- 18.3.5. CAR-T Cell Therapies Market for Other Target Antigens: Forecasted Estimates (till 2035)
- 18.4. Data Triangulation and Validation
19. CAR-T CELL THERAPY MARKET, BY TARGET INDICATION
- 19.1. Chapter Overview
- 19.2. Key Assumptions and Methodology
- 19.3. CAR-T Cell Therapies Market: Distribution by Target Indication, Current Year and 2035
- 19.3.1. CAR-T Cell Therapies Market for Multiple Myeloma: Forecasted Estimates (till 2035)
- 19.3.2. CAR-T Cell Therapies Market for Large B-Cell Lymphoma: Forecasted Estimates (till 2035)
- 19.3.3. CAR-T Cell Therapies Market for Acute Lymphoblastic Leukemia: Forecasted Estimates (till 2035)
- 19.3.4. CAR-T Cell Therapies Market for Diffuse Large B-Cell Lymphoma: Forecasted Estimates (till 2035)
- 19.3.5. CAR-T Cell Therapies Market for Diffuse Large B-Cell Lymphoma and Large B-Cell Lymphoma: Forecasted Estimates (till 2035)
- 19.3.6. CAR-T Cell Therapies Market for Acute Lymphoblastic Leukemia/ B-cell Non-Hodgkin Lymphoma: Forecasted Estimates (till 2035)
- 19.3.7. CAR-T Cell Therapies Market for Non-Hodgkin Lymphoma: Forecasted Estimates (till 2035)
- 19.3.8. CAR-T Cell Therapies Market for Mantle Cell Lymphoma: Forecasted Estimates (till 2035)
- 19.3.9. CAR-T Cell Therapies Market for Acute Myeloid Leukemia: Forecasted Estimates (till 2035)
- 19.3.10. CAR-T Cell Therapies Market for Generalized Myasthenia Gravis: Forecasted Estimates (till 2035)
- 19.3.11. CAR-T Cell Therapies Market for Renal Transplantation: Forecasted Estimates (till 2035)
- 19.3.12. CAR-T Cell Therapies Market for Gastric Adenocarcinoma: Forecasted Estimates (till 2035)
- 19.3.13. CAR-T Cell Therapies Market for Ovarian / Endometrial cancer: Forecasted Estimates (till 2035)
- 19.3.14. CAR-T Cell Therapies Market for Chronic Lymphocytic Leukemia: Forecasted Estimates (till 2035)
- 19.3.15. CAR-T Cell Therapies Market for Follicular Lymphoma: Forecasted Estimates (till 2035)
- 19.3.16. CAR-T Cell Therapies Market for Renal Cell Carcinoma: Forecasted Estimates (till 2035)
- 19.6. Data Triangulation and Validation
20. CAR-T CELL THERAPY MARKET: DISTRIBUTION BY KEY GEOGRAPHICAL REGIONS
- 20.1. Chapter Overview
- 20.2. Key Assumptions and Methodology
- 20.3. CAR-T Cell Therapy Market: Distribution by Key Geographical Regions, Current Year and 2035
- 20.3.1. CAR-T Cell Therapy Market in North America: Forecasted Estimates (till 2035)
- 20.3.2. CAR-T Cell Therapy Market for Europe: Forecasted Estimates (till 2035)
- 20.3.3. CAR-T Cell Therapy Market for Asia-Pacific: Forecasted Estimates (till 2035)
- 20.3.4. CAR-T Cell Therapy Market for Latin America: Forecasted Estimates (till 2035)
- 20.3.5. CAR-T Cell Therapy Market for Middle East and North Africa: Forecasted Estimates (till 2035)
- 20.3.6. CAR-T Cell Therapy Market for Rest of the World: Forecasted Estimates (till 2035)
- 20.4. Data Triangulation and Validation
21. CAR-T THERAPY MARKET, SALES FORECAST OF DRUGS
- 21.1. Chapter Overview
- 21.2. Key Assumptions and Methodology
- 21.3. Commercialized CAR-T Cell Therapies: Sales Forecast
- 21.3.1. Kymriah (Tisagenlecleucel-T) Sales Forecast
- 21.3.2. Yescarta (axicabtagene ciloleucel) Sales Forecast
- 21.3.3. Tecartus (Brexucabtagene Autoleucel) Sales Forecast
- 21.3.4. Abecma (Idecabtagene Vicleucel / bb211211) Sales Forecast
- 21.3.5. CARVYKTI (LCAR-B38M CAR-T / JNJ-6821845218 / Ciltacabtagene Autoleucel) Sales Forecast
- 21.3.6. BREYANZI (Lisocabtagene maraleucel, JCAR017) Sales Forecast
- 21.3.7. Carteyva (Relmacabtagene autoleucel / JWCAR0219) Sales Forecast
- 21.3.8. NexCART Sales Forecast
- 21.3.9. Fucaso (Equecabtagene Autoleucel) Sales Forecast
- 21.3.10. Inaticabtagene Autoleucel CNCT19 / HY001Sales Forecast
- 21.3.11. Zevorcabtagene autoleucel (CT053) Sales Forecast
- 21.4. Clinical CAR-T Cell Therapies: Sales Forecast
- 21.4.1. CAR-BCMA T cells Sales Forecast
- 21.4.2. CAR-T-CD19 Cells Sales Forecast
- 21.4.3. Descartes-08 Sales Forecast
- 21.4.4. Zamtocabtagene Autoleucel (MB-CART21019.1) Sales Forecast
- 21.4.5. CAR-T ddBCMA Sales Forecast
- 21.4.6. CRG-02121 cells Sales Forecast
- 21.4.7. CT041 Sales Forecast
- 21.4.8. ALLO-501A / ALLO-501 Sales Forecast
- 21.4.9. ALLO-605 Sales Forecast
- 21.4.10. Descartes-25 Sales Forecast
- 21.4.11. AUTO1 Sales Forecast
- 21.4.12. AUTO3 (CD19/2121 CAR-T) Sales Forecast
- 21.4.13. AUTO4 (CD19/2121 CAR-T) Sales Forecast
- 21.4.14. CD19-CAR-T Sales Forecast
- 21.4.15. Humanized CD19-CAR-T Sales Forecast
- 21.4.16. IM19 CAR-T Sales Forecast
- 21.4.17. CCT301 CAR-T Sales Forecast
- 21.4.18. CARCIK-CD19 Sales Forecast
- 21.4.19. CD123 CAR-T cells Sales Forecast
- 21.4.20. BCMA CAR-T Sales Forecast
- 21.4.21. CD19/CD22-CAR-T Sales Forecast
- 21.4.22. GC012F (Dual CAR-BCMA- 19) Sales Forecast
- 21.4.23. CD19/CD210-CART Sales Forecast
- 21.4.24. CD7 CAR-T Sales Forecast
- 21.4.25. Anti-FLT3 CAR-T / TAA05 Sales Forecast
- 21.4.26. Anti-ALPP CAR-T Cells Sales Forecast
- 21.4.27. WU CART 007 Sales Forecast
- 21.4.28. CTX110 Sales Forecast
- 21.4.29. TX2100-TR101 Sales Forecast
- 21.4.30. ALETA-001 Sales Forecast
- 21.4.31. PBCAR0191 Sales Forecast
- 21.5. Data Triangulation and Validation
22. CAR-T CELL THERAPY MARKET: SALES FORECAST OF LEADING PLAYERS
- 22.1. Chapter Overview
- 22.2. Key Assumptions and Methodology
- 22.3. CAR-T Cell Therapies: Leading Players
- 22.3.1. Gilead Sciences Sales Forecast
- 22.3.2. Bristol Myers Squibb Sales Forecast
- 22.3.3. Novartis Sales Forecast
- 22.3.4. Janssen Sales Forecast
- 22.3.5. JW Therapeutics Sales Forecast
- 22.4. Data Triangulation and Validation
23. PROMOTIONAL ANALYSIS
- 23.1. Chapter Overview
- 23.2. Channels Used for Promotional Campaigns
- 23.3. Summary of Product Website Analysis
- 23.4. Summary of Patient Support Services and Informative Downloads
- 23.5. Kymriah: Promotional Analysis
- 23.5.1. Drug Overview
- 23.5.2. Product Website Analysis
- 23.5.2.1. Message for Healthcare Professionals
- 23.5.2.2. Message for Patients
- 23.5.2.3. Informative Downloads
- 23.5.3. Patient Support Services
- 23.6. Yescarta: Promotional Analysis
- 23.6.1. Drug Overview
- 23.6.2. Product Website Analysis
- 23.6.2.1. Message for Healthcare Professionals
- 23.6.2.2. Message for Patients
- 23.6.2.3. Informative Downloads
- 23.6.3. Patient Support Services
- 23.7. Tecartus: Promotional Analysis
- 23.7.1. Drug Overview
- 23.7.2. Product Website Analysis
- 23.7.2.1. Message for Healthcare Professionals
- 23.7.2.2. Message for Patients
- 23.7.2.3. Informative Downloads
- 23.7.3. Patient Support Services
- 23.8. Breyanzi: Promotional Analysis
- 23.8.1. Drug Overview
- 23.8.2. Product Website Analysis
- 23.8.2.1. Message for Healthcare Professionals
- 23.8.2.2. Message for Patients
- 23.8.2.3. Informative Downloads
- 23.8.3. Patient Support Services
- 23.9. Abecma: Promotional Analysis
- 23.9.1. Drug Overview
- 23.9.2. Product Website Analysis
- 23.9.2.1. Message for Healthcare Professionals
- 23.9.2.2. Message for Patients
- 23.9.2.3. Informative Downloads
- 23.9.3. Patient Support Services
- 23.10. Carvykti: Promotional Analysis
- 23.10.1. Drug Overview
- 23.10.2. Product Website Analysis
- 23.10.2.1. Message for Healthcare Professionals
- 23.10.2.2. Message for Patients
- 23.10.2.3. Informative Downloads
- 23.10.3. Patient Support Services
24. EXECUTIVE INSIGHTS
- 24.1. Chapter Overview
- 24.2. Company A
- 24.2.1. Interview Transcript
- 24.3. Company B
- 24.3.1. Interview Transcript
- 24.4. Company C
- 24.4.1. Interview Transcript
- 24.5. Company D
- 24.5.1 Interview Transcript
- 24.6. Company E
- 24.6.1. Interview Transcript
- 24.7. Company F
- 24.7.1. Interview Transcript
- 24.8. Company G
- 24.8.1. Interview Transcript
- 24.9. Company H
- 24.9.1. Interview Transcript
- 24.10. Company I
- 24.10.1. Interview Transcript
- 24.11. Company J
- 24.11.2. Interview Transcript



















