
|
시장보고서
상품코드
1741562
<2025> 단결정(단입자) 양극재 기술개발 최신 동향 및 시장 전망(-2035)<2025> Outlook on Single-Crystal Cathode Materials: Technology Trends and Market Forecast (~2035) |
||||||
리튬이온 배터리의 고에너지 밀도화 추세에 따라, 양극재의 구조적 안정성과 수명 특성 확보가 핵심 과제로 부상하고 있습니다. 특히, 하이니켈계(Ni ≥ 80%) 삼원계 양극재(NCM, NCA)의 상용화 확대와 더불어, 그 결정 구조의 내구성을 강화할 수 있는 단결정(single crystal) 양극재 기술이 주목받고 있습니다.
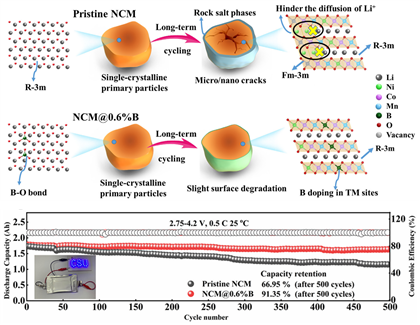
기존의 다결정(polycrystalline) 양극재는 입자 간 계면이 많아 충·방전 시 전기화학적 스트레스에 의한 균열이 발생하기 쉬우며, 전해질과의 반응성이 높아 열화가 빠르게 진행됩니다. 반면, 단결정 양극재는 단일 입자 구조로 구성되어 있어 이러한 계면 문제가 현저히 감소하며, 구조적 안정성과 수명 특성에서 우위를 가집니다. 특히, 반복적인 충·방전에서도 입자 내부 균열이나 파쇄 현상이 적어, 전기차(EV)와 같은 고사이클 응용 분야에 적합합니다.
현재 상용화된 전기차에 적용된 배터리 양극재는 수많은 금속화합물 결정이 모인 다결정 구조입니다. 그런데 이러한 양극 활물질을 일정 두께로 만드는 압연 공정 및 충방전 과정에서 입자간 균열이 발생하기가 쉽습니다. 충전과 방전이 반복될수록 소재 사이의 균열이 생겨 틈이 커집니다. 균열에 의한 소재의 파괴는 배터리 내부에서 가스발생을 증가시키고, 충방전 사이클을 감소시켜 수명감소로 이어집니다.
반면 단결정은 입자가 부서지지 않아 이런 문제가 줄어듭니다. 또한 용량을 증가시키기 위해 소재내 니켈의 함량을 증가시킴에 따라 구조적인 안정성이 낮아지고 화재 위험성이 높아지고 있어 이에 대한 해결책으로 단결정 양극재의 개발 필요성이 커지고 있습니다.
단결정 양극재는 또한, 양극재 가공 비용을 낮추고 수율도 개선할 수 있습니다. 즉, 단결정 양극재는 잔해물이 없어 불량품 발생 확률을 낮추고 수세(washing)공정을 거칠 필요가 없기 때문입니다. 수세공정은 양극재를 만들 때 반드시 필요한 공정으로 물로 불순물을 제거하는 과정입니다.
단결정 양극재가 상용화되면 하이니켈 양극재 적용도 확대될 전망입니다. 또 가스발생이 줄면 그만큼 수명이 증가하고 더 많은 활물질로 내부를 채울 수 있어 에너지밀도를 높일 수 있습니다. 이를 전기차 배터리 팩에 적용하면 지금보다 더 적은 배터리 셀 개수로 1회 충전으로 500km이상의 주행거리를 달성하고, 롱레인지 모델 등 더 긴 주행거리를 갖춘 차량 라인업을 갖출 수 있게 됩니다. 즉 원가 절감과 성능 향상을 동시에 잡을 수 있는 게임 체인저가 될 수 있는 이유입니다.
한편 단결정 양극재의 장점만 있는 것이 아니고 단점도 있습니다. 그동안 다결정 양극재 개발에 주력한 이유는 입자가 큰 단결정 소재는 초기 저항값이 높아 원하는대로 전압을 걸 수 없었습니다. 이렇게 되면 제대로 된 출력이 나오지 않아 배터리 성능을 높일 수 없는 단점이 있기 때문이었습니다.
다만 추가 공정이 필요하고 작동 전압이 커서 배터리 온도가 상승할 수 있습니다. 또한 전극공정의 일부인 압연공정에서 단결정 입자가 손상될 수 있어서 양산 초기에는 순수한 단결정이 아니라 다결정과 섞어서 생산이 이루어질 것으로 보입니다.
최근의 연구개발에서는 단결정 입자의 크기 제어 및 균일한 입자 분포 기술, 고니켈 함량에서의 결정 안정성 확보, 코팅 및 도핑 기술을 통한 표면 개질 등이 핵심 화두로 떠오르고 있습니다. 예를 들어, 단결정 NCM811, NCM90 계열 양극재에 대해 Zr, W, Al 등의 도핑 기술을 적용하여 용량 및 수명 특성을 동시에 향상시키는 시도가 활발히 이루어지고 있으며, 고온에서도 구조 안정성을 확보하는 열처리 공정 기술도 진보하고 있습니다.
또한, 상용화 관점에서는 단결정 양극재의 합성 공정 단가 절감이 중요한 과제로 지목되고 있으며, 고효율 수열합성법, 연속 플로우 반응기 기술 등을 적용한 대량생산 기술 개발이 병행되고 있습니다. 전구체 합성에서의 정밀 제어, 1차·2차 입자 형상 제어, 코팅 공정의 자동화 등이 이러한 흐름을 뒷받침하고 있습니다.
이미 중국의 업체들은 NCM523, 622등에서 단결정 양극재를 생산하고 있으며, LG화학, 에코프로비엠, 엘앤에프, 포스코 퓨처엠 등 국내 양극제조업체들도 단결정 기반의 고신뢰성 양극재 사용을 점진적으로 확대하고 있습니다. 특히, Tesla와 같은 선도 전기차 업체는 급속충전과 고온 안정성 확보를 위해 단결정 고니켈 양극재를 전략적 소재로 간주하고 있으며, 관련 협력사와의 공급 계약을 강화하고 있습니다. 현재, 단결정 양극재의 양산에 의한 시장은 중국의 소수 선두업체가 전체 시장의 70% 이상을 차지하고 있는 상황입니다.
한국의 각 양극업체의 발표에 의하면 23년부터 샘플제공을 시작으로 25년에는 대략 5만톤 이상을 생산할 것으로 SNE Research는 전망하고 있습니다. 현재 중국의 삼원계 단결정 양극재의 경우, 60%-70%가 NCM 523등 5 시리즈 단결정 비중이 가장 높으며, NCM622 등 6 시리즈 단결정 비중은 18- 25% 정도를 차지하고 있으며, Ni함량이 80%이상인 8 시리즈인 경우는 21년부터 생산비중이 증가하여 현재는 약 15%를 점유하고 있는 것으로 나타나 있으며, 이 비중은 계속 증가할 것으로 보입니다.
본 보고서의 Strong Point
① 단결정 Ni계 양극재 개발의 fundamental 및 advances까지 내용 수록
② 단결정 Ni-rich계 양극재에 대한 상세 연구동향 및 향후 전망 수록
③ 단결정 Ni-rich계 양극의 용량열화 메커니즘 연구에 대한 내용 수록
④ 단결정 NCM 양극재의 한국, 중국의 시장 전망(-2035)
⑤ 단결정 양극재 업체의 상세한 최근 개발동향 및 특허 분석
⑥ 단결정 양극재 개발에 관한 각 국의 프로그램 내용 수록
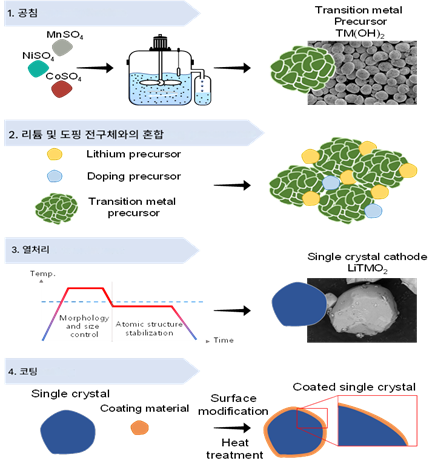
그림. 단결정(단입자) Ni 계 layered 양극재 합성 및 추가 개질 공정 모식도
목차
1. 양극재 개요
- 1.1. 양극재 개발의 역사
- 1.2. 양극재를 둘러싼 최근의 동향
- 1.2.1. 층상 산화물 양극재
- 1.2.2. 스피넬 산화물 양극재
- 1.2.3. 폴리음이온 산화물 양극재
- 1.3. 양극재별 개발 현황
- 1.3.1. 미세구조 변경
- 1.3.2. 양극 균열 제거
- 1.3.2.1. 양극 균열 제거를 위한 폴리머 코팅
- 1.3.2.2. 2차 입자 내 결정립계 개질을 통한 균열 형성 억제
- 1.3.3. One Pot Process 적용
- 1.3.4. 마이크로파 처리
2. 단결정 Ni-rich계 layered 양극재의 연구동향 및 향후 전망
- 2.1. 단결정 Ni-rich layered 소재 연구 필요성
- 2.1.1. Ni-rich layered소재의 필요한 이유 (장점)
- 2.1.2. Ni계 layered 양극소재의 열화 메커니즘
- 2.1.3. Ni계 layered 양극소재의 단결정화 (단입자화)의 필요성
- 2.2. 단결정 양극재 정의
- 2.3. 단결정 양극재 기술 개발 현황
- 2.3.1 단입자 Ni계 layered 소재 합성 연구
- 2.3.2 단입자 Ni계 layered 소재 합성을 위한 소결 방법 연구
- 2.3.3. 단입자 Ni계 layered 소재 성능 개선을 위한 소재 개질 연구
- 2.3.3.1 표면 코팅 연구
- 2.3.3.2. 원소 치환 연구 (도핑)
- 2.3.3.2.1. 단일 도핑
- 2.3.3.2.2. 듀얼 도핑
- 2.3.3.3. 전해액 최적화 연구
- 2.3.4. 단입자 Ni계 layered 소재 활용 방안
- 2.3.4.1. Ni계 layered 소재의 단입자화의 전극 설계 관점에서의 장점
- 2.3.4.2. Ni계 layered 소재의 단입자화의 전극 설계 관점에서의 단점
- 2.3.4.3. Ni계 layered 소재의 단입자화의 문제점을 해결하기 위한 연구
- 2.4. 소재 단입자를 통한 개선점
- 2.4.1. 입자 깨어짐 특성 완화
- 2.4.4.1. 전극 제작 공정에서의 프레스 단계
- 2.4.2. 충방전 과정에서의 입자 깨어짐
- 2.4.3. 비표면적 감소를 통한 표면 열화의 양적인 감소
- 2.4.4. 에너지 밀도 증가
- 2.4.5. 수세공정 생략 가능
- 2.4.1. 입자 깨어짐 특성 완화
- 2.5. 현 단결정 양극재 기술 개발의 한계 및 이를 해결하기 위한 연구
- 2.5.1. 합성 조건 최적화의 어려움으로 인한 소재 결정 구조의 열화
- 2.5.2. 입자 사이즈 한계
3. 단결정 Ni계 양극재 개발: Fundamental 및 advances
- 3.1. 개요
- 3.2. Ni계 양극재
- 3.2.1. 화학구조
- 3.2.2. 전자구조
- 3.3. Ni계 층상 산화물의 과제
- 3.3.1. 합성의 어려움
- 3.3.2. 구조적 불안정성
- 3.3.3. 화학적 불안정성
- 3.3.4. 기계적 성능 저하
- 3.3.5. 안전성 문제
- 3.4. 단결정 Ni계 층상 산화물의 유래
- 3.4.1. 단결정 양극 소재의 입자 조정 효과 및 성능 비교 분석
- 3.4.2. 단결정 소재를 활용한 배터리 성능 개선 전략
- 3.4.3. 배터리 소재에서의 단결정 개념과 장점
- 3.5. 단결정 Ni계 층상 산화물의 합성
- 3.5.1. 합성 방법
- 3.5.2. 단결정 양극 활물질의 합성 및 특성 연구
- 3.5.3. 다양한 고체 형성 메커니즘 및 단결정 산화물 제조 방법
- 3.6. 단결정과 다결정 재료의 비교 연구
- 3.6.1. 단결정 양극재의 배터리 안전성 및 전기화학적 성능
- 3.7. 단결정 Ni기반 양극재의 최신 공정
- 3.7.1. 도핑과 표면 코팅
- 3.7.2. 기계적 연구
- 3.8. 결과 및 결론
4. 단결정 Ni-rich NCM 양극의 용량열화 메커니즘 연구
- 4.1. 개요
- 4.2. Ni-rich 단결정, 다결정 양극의 기본특성 평가
- 4.2.1. 단결정, 다결정 양극재 합성
- 4.2.2. 단결정, 다결정 양극재 조성 및 분석
- 4.2.3. 단결정, 다결정 양극재의 전기화학적 특성
- 4.2.3.1. 단결정, 다결정 양극재의 속도 특성 및 열화 메커니즘 비교 분석
- 4.2.4. 단결정, 다결정 양극재의 구조적 응력분석
- 4.2.5. 단결정, 다결정 양극재의 in-situ XRD 분석
- 4.2.6. 단결정, 다결정 양극재의 TEM 분석
- 4.2.7. 결과 및 결론
5. 단결정 NCM 양극재의 합성 및 개질: Growth mechanism
- 5.1. 개요
- 5.2. 성장 메커니즘(NCM양극에 대한 고려사항)
- 5.2.1. 단결정 NCM 양극 성장의 열역학 및 성장 메커니즘
- 5.3. Solid state 반응
- 5.4. 고체-액체 유변학적 반응
- 5.5. 용융염 Flux에서의 결정 성장
- 5.6. Modification of morphology
- 5.6.1. 모양 제어
- 5.6.2. Facet의 조절
- 5.6.3. 결론
6. 단결정 Ni-rich 양극재의 입자제어 (소결 조제 적용)
- 6.1. 개요
- 6.2. 실험: 전략적 접근
- 6.3. 실험 결과
- 6.3.1. 결정성장 촉진을 위한 소결 조제의 최적화
- 6.3.2. 결정성장 메카니즘
- 6.3.3. Ni-rich 단결정 양극의 구조
- 6.3.4. Ni-rich 단결정 양극의 성능
- 6.3.4.1. 고밀도 전극 설계를 위한 미세구조 제어 전략
- 6.3.4.2. 파우치 셀 성능 평가 및 특성 분석
- 6.4. 소결 조제의 적용 결과
7. 단결정 NCM 양극재의 올 건식 합성(All Dry Synthesis)
- 7.1. 개요
- 7.1.1. 단결정 Ni-rich NMC 양극재의 제조 공정 고찰
- 7.2. 건식 합성
- 7.3. 건식 합성 결과 및 논의
- 7.3.1. 전구체 구조 및 형태
- 7.3.2. NCM 형성에 대한 소결 조건의 영향
- 7.3.2.1. 단결정-NCM1-3의 전기화학적 성능
- 7.3.3. 볼 밀링 전구체의 단결정 NCM
- 7.3.4. 결론
8. 단결정 NCM523 양극재의 One Spot Synthesis
- 8.1. 개요
- 8.2. NCM523의 합성
- 8.3. 재료의 특성화(Characterization)
- 8.4. 전기화학적 특성
- 8.5. 실험결과 및 논의
- 8.5.1. 양극재 합성 결과물 분석
- 8.5.2. 양극재의 전기화학적 특성
- 8.5.3. 결론
9. 단결정 NCM9073 양극재의 합성 및 개질
- 9.1. 개요
- 9.2. 실험 설계 및 분석법
- 9.2.1. 단결정 NCM9073 소재 합성 및 개질 방법
- 9.2.2. 물성 분석
- 9.2.3. 전기화학적 성능 평가
- 9.3. Zn 표면 집중 도핑 결과 및 해석
- 9.3.1. Zn 표면 집중 도핑 단결정 NCM9073 물성 분석 결과 해석
- 9.3.2. Zn 표면 집중 도핑 단결정 NCM9073 전기화학적 성능 평가
- 9.3.3. 고온 (60℃) 환경에서의 전기화학적 성능 및 물성 변화
- 9.3.4. Zn 표면 집중 도핑 단결정 NCM9073 열 안정성 평가
10. 단결정 양극재 시장 규모 및 전망
- 10.1. 단결정 양극재 시장 개요 및 성장 동인
- 10.1.1. 단결정 양극재 지역별 시장 점유율
- 10.1.2. 단결정 양극재 유형별 시장 점유율
- 10.1.3. 단결정 양극재 주요 응용 분야/시장동향 기회/제약 및 과제
- 10.1.4. 단결정 양극재 시장 수요 변화와 점유율 변동
- 10.2. 중국 단결정 하이니켈 삼원계 양극재 산업 시장 규모 및 전망
- 10.2.1. 중국 단결정 하이니켈 삼원계 양극재 산업 시장 개요
- 10.2.2. 중국 단결정 하이니켈 3원계 양극재 시장 규모 분석
- 10.2.3. 중국 하이니켈 3원계 단결정 양극재 수요 및 공급 분석
- 10.2.4. 단결정 하이니켈 3원계 양극재 글로벌 시장과 중국 시장 비교
- 10.3. 한국-중국 삼원계 단결정 생산량 전망(2019-2035)
- 10.4. 한국 삼원계 단결정 양극재 생산량 및 시장 전망(2019-2035)
- 10.5. 중국 삼원계 단결정 양극재 생산량 및 시장 전망(2019-2035)
- 10.6. 삼원계 단결정 양극재 평균가격(ASP) 추이(2019-2035)
- 10.7. 중국 삼원계 단결정 양극재별 점유율 전망(2019-2035)
- 10.8. 중국 삼원계 단결정 양극재별 시장 전망(2019-2035)
11. 단결정 양극재 개발 각국 Program
- 11.1. 미국 DOE Program
- 11.1.1. Ni-rich 단결정 양극재의 초고속 수열법에 의한 생산
- 11.1.2. Ni-rich 고성능 단결정 양극재의 scale-up
- 11.1.3. 고성능 전고체 LIB를 구현하는 단결정 양극재
- 11.2. EU Program
- 11.2.1. LIBEST2 Project
- 11.2.2. FutureCat Project
- 11.3. 한국 국가과제 Program
- 11.4. 일본 국가과제 Program
- 11.4.1. 저결정 제어에 의한 고에너지 밀도 양극 재료의 창조
- 11.4.2. Solving problems through material design & creation
12. 단결정 양극재 업체 특허 분석
- 12.1. Tesla
- 12.2. LG Chem
- 12.3. SM Lab
- 12.4. Nano One Materials
- 12.5. 포스코 퓨처엠
- 12.6. 코스모 신소재
- 12.7. 엘엔에프
- 12.8. Easpring
- 12.9. BASF Shan Shan
- 12.10. GEM
- 12.11. XTC(Xiamen Tungsten)
- 12.12. Henan Kelong
- 12.13. 현대/기아자동차
- 12.14. 6K Inc.
- 12.15. Dynanonic
- 12.16. Suzhou Long Power
- 12.17. Fengchao Energy
- 12.18. Ecopro BM
- 12.19. Umicore
13. 단결정 양극재 업체 동향
- 13.1. LG Chem
- 13.2. 포스코 퓨처엠
- 13.3. 에코프로비엠
- 13.4. 금양(SM Lab)
- 13.5. 엘앤에프(L&F)
- 13.6. 코스모 신소재
- 13.7. Umicore
- 13.8. 스미토모 금속광산
- 13.9. Toda 공업
- 13.10. Guizhou Zhenhua E-chem (ZEC)
- 13.11. Chanyuan Lico
- 13.12. Ronbay
- 13.13. XTC (Xiamen Tungsten)
- 13.14. Tianjin B&M
- 13.15. Easpring
- 13.16. Reshane
- 13.17. Yibin Libode
- 13.18. Wanxing 123
- 13.19. GEM
References
(주말 및 공휴일 제외)













