
|
시장보고서
상품코드
1705641
바이오시밀러 수탁 제조 시장 : 제품 유형별, 용도별, 유형별, 서비스 유형별, 최종 사용자별, 지역별(2025-2032년)Biosimilar Contract Manufacturing Market, By Product Type, By Application, By Type, By Service Type , By End User, By Geography |
||||||
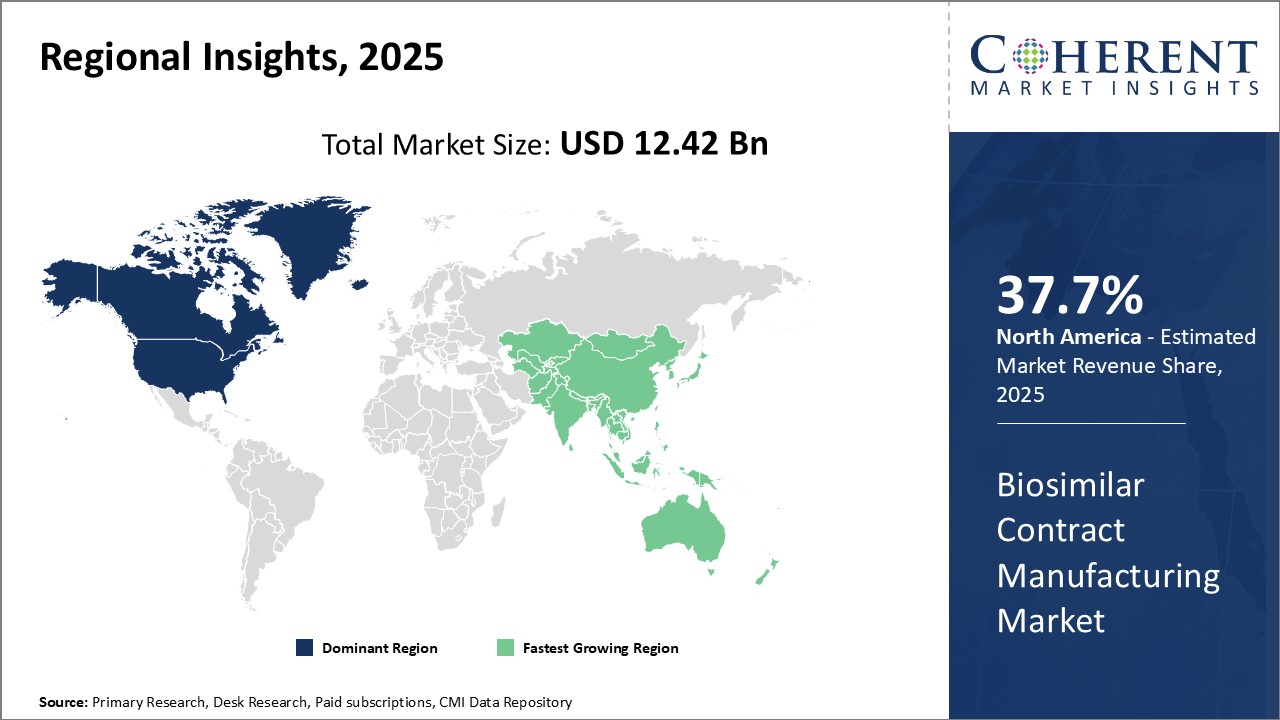
세계의 바이오시밀러 수탁 제조 시장은 2025년 124억 2,000만 달러로 추정되며, 2032년까지 353억 5,000만 달러에 이를 것으로 예측되며, 2025년부터 2032년까지 연평균 성장률(CAGR) 16.1%로 성장할 전망입니다.
| 보고 범위 | 보고서 세부정보 | ||
|---|---|---|---|
| 기준연도 | 2024년 | 2025년 시장 규모 | 124억 2,000만 달러 |
| 실적 데이터 | 2020-2024년 | 예측 기간 | 2025-2032년 |
| 예측 기간: 2025-2032년 CAGR: | 16.10% | 2032년 가치 예측 | 353억 5,000만 달러 |
바이오시밀러는 이미 승인된 생물학적 제제(참조 제제 또는 오리지네이터 제제라고 함)와 치료 효과가 매우 유사하도록 설계된 생물학적 제제입니다. 제조 수탁기관(CMO)은 제약회사가 바이오시밀러의 제조 공정을 개발하여 규모를 확대함으로써 엄격한 품질기준에 적합한 바이오시밀러의 제조를 지원합니다.
시장 역학:
세계의 바이오시밀러 수탁 제조 시장의 성장을 견인하고 있는 것은 생물 제제의 특허 만료에 의한 저렴한 바이오시밀러에 대한 수요 증가와, 비용 대비 효과의 높이에 의한 바이오시밀러에 대한 주목의 높아입니다. 그러나, 바이오시밀러 제조 시설의 설치에는 높은 설비 투자가 필요합니다. 한편, 아시아태평양과 라틴아메리카 등의 신흥 시장은 헬스 케어 인프라가 정비되어 환자가 바이오시밀러에 접근하기 쉬워지고 있으며, 큰 수익의 가능성을 지니고 있습니다.
본 조사의 주요 특징
본 보고서에서는 세계의 바이오시밀러 수탁 제조 시장을 상세하게 분석하여 2024년을 기준연도로 한 예측기간 중(2025-2032년) 시장 규모(10억 달러)와 연간 평균 성장률(CAGR%)을 제공합니다.
또한 다양한 부문에 걸친 잠재적인 수익 기회를 밝히고 이 시장의 매력적인 투자 제안 행렬을 설명합니다.
또한 시장 성장 촉진요인, 억제요인, 기회, 신제품의 상시 및 승인, 시장 동향, 지역별 전망, 주요 기업이 채용하는 경쟁 전략 등에 관한 중요한 고찰도 제공합니다.
세계 바이오시밀러 수탁 제조시장의 주요 기업을 기업 하이라이트, 제품 포트폴리오, 주요 하이라이트, 실적, 전략 등의 파라미터를 기반으로 프로파일하고 있습니다.
본 조사의 대상이 되는 주요 기업으로는 Biocon, Amgen, Pfizer, Boehringer Ingelheim, Lonza, Catalent, Wuxi Biologics, AbbVie, Merck KGaA, Rentschler Biopharma, Almac Group, Fujifilm Diosynth Biotechnologies, Evonik Industries, Avid Bioservices 등이 포함됩니다.
이 보고서의 인사이트를 통해 마케팅 담당자와 기업 경영진은 향후 제품 상시, 타이핑, 시장 확대, 마케팅 전술에 대한 정보를 바탕으로 의사 결정을 내릴 수 있습니다.
세계 바이오시밀러 수탁 제조 시장 보고서는 투자자, 공급업체, 제품 제조업체, 유통업체, 신규 참가자, 재무 분석가 등 이 업계의 다양한 이해관계자를 지원합니다.
이해관계자들은 세계 바이오시밀러 수탁 제조 시장 분석에 사용되는 다양한 전략 매트릭스를 통해 의사 결정이 용이해집니다.
목차
제1장 조사의 목적과 전제조건
- 조사 목적
- 전제조건
- 약어
제2장 시장 전망
- 보고서 설명
- 시장 정의와 범위
- 주요 요약
제3장 시장 역학, 규제, 동향 분석
- 시장 역학
제4장 세계의 바이오시밀러 수탁 제조 시장 : 제품 유형별, 2020-2032년
- 재조합 비글리코실화 단백질
- 재조합 당화 단백질
제5장 세계의 바이오시밀러 수탁 제조 시장 : 용도별, 2020-2032년
- 종양학
- 당뇨병
- 감염증
- 만성질환 및 자가면역질환
- 혈액 질환
- 성장 호르몬 결핍증
제6장 세계의 바이오시밀러 수탁 제조 시장 : 유형별, 2020-2032년
- 포유류 세포에 의한 제조
- 미생물에 의한 제조
제7장 세계의 바이오시밀러 수탁 제조 시장 : 서비스 유형별, 2020-2032년
- 업스트림 처리
- 다운스트림 처리
- 바이오시밀러 시험
- 바이오 검정(in vitro/in vivo)
- 프로세스 개발
- 충전 및 마무리
제8장 세계의 바이오시밀러 수탁 제조 시장 : 최종 사용자별, 2020-2032년
- 바이오의약품기업
- 수탁제조기관(CMO)
- 기타
제9장 세계의 바이오시밀러 수탁 제조 시장 : 지역별, 2020-2032년
- 북미
- 라틴아메리카
- 유럽
- 아시아태평양
- 중동
- 아프리카
제10장 경쟁 구도
- Samsung Biologics
- Biocon
- Amgen
- Pfizer
- Boehringer Ingelheim
- Lonza
- Catalent
- Wuxi Biologics
- AbbVie
- Merck KGaA
- Rentschler Biopharma
- Almac Group
- Fujifilm Diosynth Biotechnologies
- Evonik Industries
- Avid Bioservices
제11장 분석가 추천
- 운명의 원
- 애널리스트의 견해
- 일관성 있는 기회 맵
제12장 참고문헌과 조사방법
- 참고문헌
- 조사 방법
- 출판사에 대해
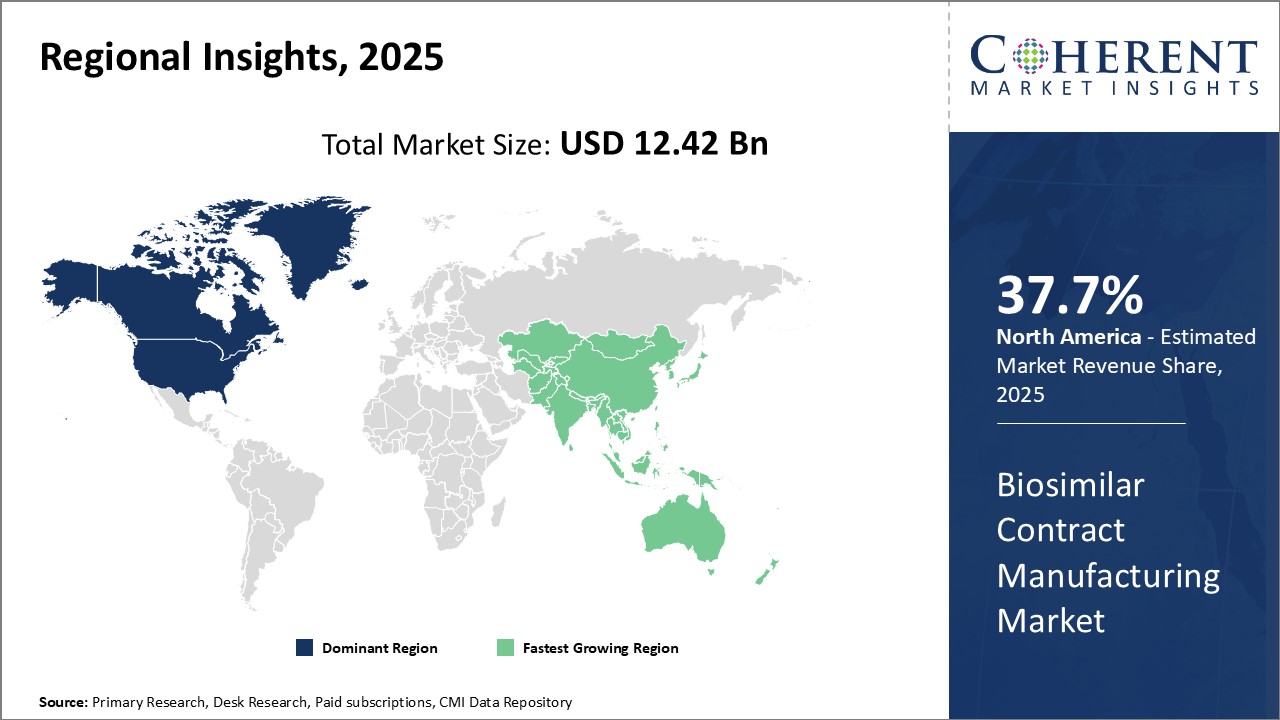
Global Biosimilar Contract Manufacturing Market is estimated to be valued at USD 12.42 Bn in 2025 and is expected to reach USD 35.35 Bn by 2032, growing at a compound annual growth rate (CAGR) of 16.1% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 12.42 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 16.10% | 2032 Value Projection: | USD 35.35 Bn |
Biosimilars are biological products designed to have highly similar therapeutic effect to an already approved biologic product, known as the reference product or originator product. With the patent expiry of several blockbuster biologics, biosimilars provide an opportunity for patient access to more affordable healthcare. Contract manufacturing organizations (CMOs) aid pharmaceutical companies in manufacturing biosimilars to exacting quality standards through process development and scaling up. Offloading biosimilar manufacturing to CMOs allows drug makers to focus on core competencies such as R&D.
Market Dynamics:
The global biosimilar contract manufacturing market growth is driven by the growing demand for affordable biosimilars due to patent expiration of biologics and increasing focus on biosimilars due to their cost-effectiveness. However, high capital investment requirements for setting up biosimilar manufacturing facilities and stringent regulatory standards for biosimilars pose challenges to market players. On the other hand, emerging markets, such as Asia Pacific and Latin America, present significant revenue potential with improving healthcare infrastructure and patient access to biosimilars in these regions. Contract manufacturers have opportunities to provide end-to-end services from cell line development to commercial manufacturing of biosimilars and help expand market reach.
Key Features of the Study:
This report provides in-depth analysis of the global biosimilar contract manufacturing market, and provides market size (USD Bn) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
It profiles key players in the global biosimilar contract manufacturing market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
Key companies covered as a part of this study include Biocon, Amgen, Pfizer, Boehringer Ingelheim, Lonza, Catalent, Wuxi Biologics, AbbVie, Merck KGaA, Rentschler Biopharma, Almac Group, Fujifilm Diosynth Biotechnologies, Evonik Industries, and Avid Bioservices
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
The global biosimilar contract manufacturing market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global biosimilar contract manufacturing market
Market Segmentation
- Product Type Insights (Revenue, USD Bn, 2020 - 2032)
- Recombinant Non-glycosylated Proteins
- Recombinant Glycosylated Proteins
- Application Insights (Revenue, USD Bn, 2020 - 2032)
- Oncology
- Diabetes
- Infectious Diseases
- Chronic and Autoimmune Disorders
- Blood Disorders
- Growth Hormonal Deficiency
- Type Insights (Revenue, USD Bn, 2020 - 2032)
- Mammalian Manufacturing
- Microbial Manufacturing
- Service Type Insights (Revenue, USD Bn, 2020 - 2032)
- Upstream Processing
- Downstream Processing
- Biosimilarity testing
- Bioassay (in vitro/in vivo)
- Process Development
- Fill & Finish
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Biopharmaceutical Companies
- Contract Manufacturing Organizations (CMOs)
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players Insights
- Biocon
- Amgen
- Pfizer
- Boehringer Ingelheim
- Lonza
- Catalent
- Wuxi Biologics
- AbbVie
- Merck KGaA
- Rentschler Biopharma
- Almac Group
- Fujifilm Diosynth Biotechnologies
- Evonik Industries
- Avid Bioservices
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Global Biosimilar Contract Manufacturing Market, By Product Type
- Global Biosimilar Contract Manufacturing Market, By Application
- Global Biosimilar Contract Manufacturing Market, By Type
- Global Biosimilar Contract Manufacturing Market, By Service Type
- Global Biosimilar Contract Manufacturing Market, By End User
- Global Biosimilar Contract Manufacturing Market, By Region
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Driver
- Restraint
- Opportunity
- Impact Analysis
- Key Developments
- Regulatory Scenario
- Product Launches/Approvals
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
- Industry Trends
4. Global Biosimilar Contract Manufacturing Market, By Product Type, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Recombinant Non-glycosylated Proteins
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Recombinant Glycosylated Proteins
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
5. Global Biosimilar Contract Manufacturing Market, By Application, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Oncology
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Diabetes
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Infectious Diseases
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Chronic and Autoimmune Disorders
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Blood Disorders
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Growth Hormonal Deficiency
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
6. Global Biosimilar Contract Manufacturing Market, By Type, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Mammalian Manufacturing
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Microbial Manufacturing
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
7. Global Biosimilar Contract Manufacturing Market, By Service Type, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Upstream Processing
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Downstream Processing
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Biosimilarity testing
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Bioassay (in vitro/in vivo)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Process Development
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Fill & Finish
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
8. Global Biosimilar Contract Manufacturing Market, By End User, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Biopharmaceutical Companies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Contract Manufacturing Organizations (CMOs)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
9. Global Biosimilar Contract Manufacturing Market, By Region, 2020 - 2032, Value (USD Bn)
- Introduction
- Market Share (%) Analysis, 2025, 2028 & 2032, Value (USD Bn)
- Market Y-o-Y Growth Analysis (%), 2021 - 2032, Value (USD Bn)
- Regional Trends
- North America
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Service Type , 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- U.S.
- Canada
- Latin America
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Service Type , 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Service Type , 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Service Type , 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Service Type , 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Service Type , 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country/Region, 2020 - 2032, Value (USD Bn)
- South Africa
- North Africa
- Central Africa
10. Competitive Landscape
- Samsung Biologics
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Biocon
- Amgen
- Pfizer
- Boehringer Ingelheim
- Lonza
- Catalent
- Wuxi Biologics
- AbbVie
- Merck KGaA
- Rentschler Biopharma
- Almac Group
- Fujifilm Diosynth Biotechnologies
- Evonik Industries
- Avid Bioservices
11. Analyst Recommendations
- Wheel of Fortune
- Analyst View
- Coherent Opportunity Map
12. References and Research Methodology
- References
- Research Methodology
- About us



















