
|
시장보고서
상품코드
1672840
전자 임상시험 마스터 파일(eTMF) 시장 : 배포 모드별, 기능별, 최종 사용자별, 지역별Electronic Trial Master File (eTMF) Market, By Deployment Mode, By Functionality, By End User, By Geography |
||||||
세계의 전자 임상시험 마스터 파일(eTMF) 시장은 2025년에는 20억 9,000만 달러, 2032년에는 48억 1,000만 달러에 이르고, 2025-2032년의 CAGR은 12.6%를 나타낼 전망입니다.
| 보고 범위 | 보고서 세부정보 | ||
|---|---|---|---|
| 기준연도 | 2024년 | 2025년 시장 규모 | 20억 9,000만 달러 |
| 실적 데이터 | 2020-2024년 | 예측 기간 | 2025-2032년 |
| 예측 기간(2025-2032년) CAGR: | 12.60% | 2032년 가치 예측 | 48억 1,000만 달러 |

전자 임상시험 마스터 파일(eTMF) 시장의 세계 성장은 업계 전반에 걸쳐 임상시험 프로세스가 확대되고 있다는 배경에 있습니다. eTMF는 문서 검색 및 보관을 용이하게 하기 위해 설계된 디지털, 전자 형식으로 임상시험과 관련된 문서 관리를 용이하게 하는 통합 시스템을 말합니다. 중요한 임상시험 관련 문서를 조직적으로 관리하고 임상시험의 수명주기 전반에 걸쳐 가시성을 향상시킬 수 있습니다. 임상시험 프로세스가 복잡해지고 규제가 강화되는 가운데 컴플라이언스와 모니터링의 강화를 확보하기 위해 임상시험을 실시하는 조직은 eTMF 솔루션에 대한 큰 수요가 예상됩니다. 시장 성장은 규제 준수 요건, eTMF 비용 효율성, 의약품 개발에서 R&D 투자 증가와 같은 요인으로 인해 발생합니다.
시장 역학 :
임상시험 문서화 및 데이터 관리에 대한 엄격한 규제 지침은 각 지역에서 eTMF(전자 시험 관련 문서) 시장의 세계 성장을 가속할 수 있습니다. 규제 기관은 데이터 무결성을 보장하기 위해 전자 아카이브를 통한 적절한 문서 관리를 강조합니다. 신약 개발을 위한 제약 기업 및 생명 공학 기업의 R&D 비용 증가도 시장 성장을 가속할 수 있습니다. 그러나 특히 중소기업의 경우 eTMF 시스템의 도입 비용이 높아 시장 성장을 방해할 수 있습니다. eTMF의 비용 이점과 문서 보안 및 접근성 향상은 새로운 성장 기회를 초래할 수 있습니다. 벤더는 또한 맞춤형 기술과 클라우드 기반 솔루션에 집중하고 접근성을 더욱 향상시키고 운영 비용을 줄이기 위해 노력하고 있습니다.
본 조사의 주요 특징
본 보고서에서는 세계의 전자 임상시험 마스터 파일(eTMF) 시장을 상세하게 분석하여 2024년을 기준연도로 한 예측기간(2025년-2032년) 시장 규모 및 CAGR을 제공합니다.
또한 다양한 부문에 걸친 잠재적인 수익 기회를 밝히고 이 시장의 매력적인 투자 제안 매트릭스를 설명합니다.
또한 시장 성장 촉진요인, 억제요인, 기회, 신제품 출시 및 승인, 시장 동향, 지역별 전망, 주요 기업이 채용하는 경쟁 전략 등에 관한 중요한 고찰도 제공합니다.
세계 eTMF(전자 시험 관련 문서) 시장에서 주요 기업 프로파일을 기업 하이라이트, 제품 포트폴리오, 주요 하이라이트, 재무 실적, 전략 등의 매개변수를 기반으로 제공합니다.
본 연구의 대상이 되는 주요 기업은 Veeva Systems Inc., Medidata Solutions, Inc., Oracle Corporation, Parexel International Corporation, IBM Watson Health, DrugDev(현재 Veeva의 일부), MasterControl, Inc., Bioclinica, Inc. 등이 있습니다.
이 보고서의 통찰을 통해 마케팅 담당자와 기업 경영진은 향후 제품 출시, 타이핑, 시장 확대, 마케팅 전술에 대한 정보를 바탕으로 의사 결정을 내릴 수 있습니다.
세계 eTMF(전자 시험 관련 문서) 시장 보고서는 투자자, 공급업체, 제품 제조업체, 유통업체, 신규 참가자, 재무 분석가 등 이 업계의 다양한 이해관계자를 지원합니다.
이해관계자는 세계 eTMF(전자 시험 관련 문서) 시장을 분석하는데 사용되는 다양한 전략 매트릭스를 통해 의사결정을 용이하게 할 수 있습니다.
목차
제1장 조사의 목적과 전제조건
- 조사 목적
- 전제조건
- 약어
제2장 시장 전망
- 보고서 설명
- 시장 정의와 범위
- 주요 요약
제3장 시장 역학, 규제, 동향 분석
- 시장 역학
- 성장 촉진요인
- 억제요인
- 시장 기회
- 규제 시나리오
- 업계 동향
- 합병과 인수
- 신시스템 도입, 승인
- COVID-19 팬데믹의 영향
제4장 세계의 전자 임상시험 마스터 파일(eTMF) 시장, 배포 모드별, 2020년-2032년
- 클라우드 기반
- 온프레미스
제5장 세계의 전자 임상시험 마스터 파일(eTMF) 시장, 기능별, 2020년-2032년
- 문서 관리
- 워크플로우 관리
- 보고서 및 분석
- 기타
제6장 세계의 전자 임상시험 마스터 파일(eTMF) 시장, 최종 사용자별, 2020년-2032년
- 제약회사
- 바이오테크놀러지 기업
- 수탁연구기관(CRO)
- 학술연구기관
제7장 세계의 전자 임상시험 마스터 파일(eTMF) 시장, 지역별, 2020년-2032년
- 북미
- 유럽
- 아시아태평양
- 라틴아메리카
- 중동
- 아프리카
제8장 경쟁 구도
- 기업 프로파일
- Veeva Systems Inc.
- Medidata Solutions, Inc.
- Oracle Corporation
- Parexel International Corporation
- IBM Watson Health
- DrugDev(now part of Veeva)
- MasterControl, Inc.
- ArisGlobal LLC
- Dassault Systemes
- Trial Interactive
- Signant Health
- Forte Research Systems, Inc.
- Axiom Real-Time Metrics
- eClinical Solutions, LLC
- Bioclinica, Inc.
제9장 분석가 추천
- 기회
- 애널리스트의 견해
- Coherent Opportunity Map
제10장 조사 방법
- 참고문헌
- 조사 방법
Global Electronic Trial Master File (eTMF) Market is estimated to be valued at USD 2.09 Bn in 2025 and is expected to reach USD 4.81 Bn by 2032, growing at a compound annual growth rate (CAGR) of 12.6% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.09 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.60% | 2032 Value Projection: | USD 4.81 Bn |
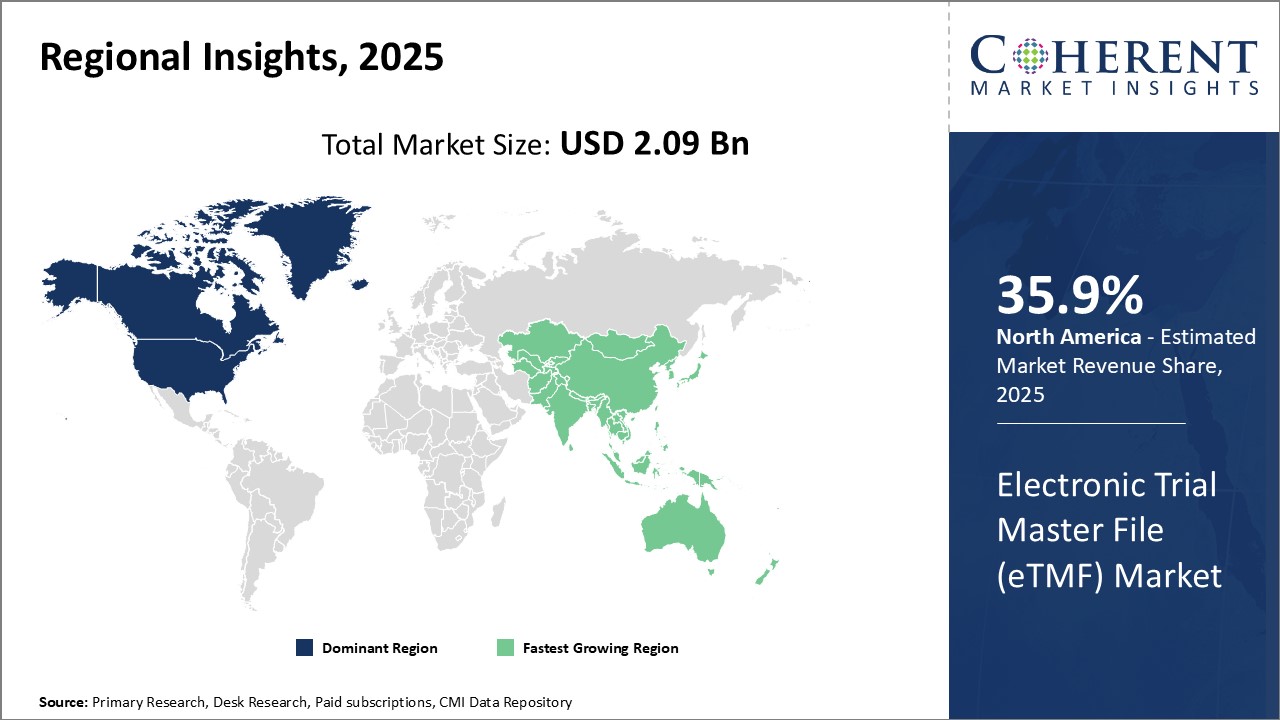
Global electronic trial master file (eTMF) market growth is driven by growing clinical trial processes across industries. eTMF refers to an integrated system that facilitates management of documents related to clinical trials in a digital/electronic format designed for ease of retrieval and archiving of documents. It helps maintain crucial trial-related documents in an organized manner and improve visibility throughout the lifecycle of a clinical trial. With increasing complexities in clinical trial processes and stricter regulations, there will be huge demand for eTMF solutions among organizations conducting trials to ensure compliance and improved oversight. The market growth is driven by factors such as regulatory compliance requirements, cost-efficiency of eTMF, and increasing R&D investments in drug development.
Market Dynamics:
Stringent regulatory guidelines around clinical trial documentation and data management across regions can drive the global electronic trial master file (eTMF) market growth. Regulatory bodies emphasize on maintaining proper documentation with electronic archiving to ensure data integrity. Rising R&D expenditure of pharmaceutical and biotechnology companies on new drug development can also drive the market growth. However, high implementation costs of eTMF systems especially for small and mid-sized companies can hamper the market growth. Cost benefits of eTMF along with better document security and accessibility can offer new growth opportunities. Vendors are also focusing on customizable technologies and cloud-based solutions to further enhance accessibility and lower operational costs.
Key features of the study:
This report provides in-depth analysis of the global electronic trial master file (eTMF) market, and provides market size (US$ Bn) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
It profiles key players in the global electronic trial master file (eTMF) market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
Key companies covered as a part of this study include Veeva Systems Inc., Medidata Solutions, Inc., Oracle Corporation, Parexel International Corporation, IBM Watson Health, DrugDev (now part of Veeva), MasterControl, Inc., ArisGlobal LLC, Dassault Systemes, Trial Interactive, Signant Health, Forte Research Systems, Inc., Axiom Real-Time Metrics, eClinical Solutions, LLC, and Bioclinica, Inc.
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
Global electronic trial master file (eTMF) market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global electronic trial master file (eTMF) market
Detailed Segmentation-
- By Deployment Mode Insights (Revenue, US$ Bn, 2020 - 2032)
- Cloud-based
- On-premises
- By Functionality Insights (Revenue, US$ Bn, 2020 - 2032)
- Document Management
- Workflow Management
- Reporting and Analytics
- Others
- By End User Insights (Revenue, US$ Bn, 2020 - 2032)
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic Research Institutions
- Regional Insights (Revenue, US$ Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players Insights
- Veeva Systems Inc.
- Medidata Solutions, Inc.
- Oracle Corporation
- Parexel International Corporation
- IBM Watson Health
- DrugDev (now part of Veeva)
- MasterControl, Inc.
- ArisGlobal LLC
- Dassault Systemes
- Trial Interactive
- Signant Health
- Forte Research Systems, Inc.
- Axiom Real-Time Metrics
- eClinical Solutions, LLC
- Bioclinica, Inc.
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Market Snippet, By Deployment Mode
- Market Snippet, By Functionality
- Market Snippet, By End User
- Market Snippet, By Region
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Drivers
- Restraints
- Market Opportunities
- Regulatory Scenario
- Industry Trend
- Merger and Acquisitions
- New System Launches/Approvals
- Impact of COVID-19 Pandemic
4. Global Electronic Trial Master File (eTMF) Market, By Deployment Mode, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- Segment Trends
- Cloud-based
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- On-premises
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
5. Global Electronic Trial Master File (eTMF) Market, By Functionality, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- Segment Trends
- Document Management
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Workflow Management
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Reporting and Analytics
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Others
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
6. Global Electronic Trial Master File (eTMF) Market, By End User, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- Segment Trends
- Pharmaceutical Companies
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Biotechnology Companies
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Contract Research Organizations (CROs)
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Academic Research Institutions
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
7. Global Electronic Trial Master File (eTMF) Market, By Region, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, By Region, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- North America
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- U.S.
- Canada
- Europe
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- China
- India
- Japan
- ASEAN
- Australia
- South Korea
- Rest of Asia Pacific
- Latin America
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Middle East
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- Israel
- GCC Countries
- Rest of the Middle East
- Africa
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country/Region, 2020-2032, (US$ Bn)
- South Africa
- North Africa
- Central Africa
8. Competitive Landscape
- Company Profiles
- Veeva Systems Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Medidata Solutions, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Oracle Corporation
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Parexel International Corporation
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- IBM Watson Health
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- DrugDev (now part of Veeva)
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- MasterControl, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- ArisGlobal LLC
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Dassault Systemes
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Trial Interactive
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Signant Health
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Forte Research Systems, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Axiom Real-Time Metrics
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- eClinical Solutions, LLC
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Bioclinica, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Veeva Systems Inc.
9. Analyst Recommendations
- Wheel of Fortune
- Analyst View
- Coherent Opportunity Map
10. Research Methodology
- References
- Research Methodology
- About us and Sales Contact
















