
|
시장보고서
상품코드
1877352
전자 임상시험 마스터 파일(eTMF) 시스템 시장 : 제공별, 기능별, 치료 영역별, 최종 사용자별, 시험 단계별, 지역별 예측(-2030년)Electronic Trial Master File (eTMF) Systems Market by Offering (Integrated, Standalone), Function (Analytic, Compliance, Workflow, Audit), Therapeutic (Onco, Cardio, Neuro), End User (Pharma, Biotech, MedDevice), Phase, Region - Global Forecast to 2030 |
||||||
세계의 전자 임상시험 마스터 파일(eTMF) 시스템 시장 규모는 예측 기간 동안 12.8% 높은 CAGR을 나타내며, 2025년 13억 6,000만 달러에서 2030년에는 24억 9,000만 달러에 이를 것으로 전망됩니다.
본 시장은 효율적인 임상시험 관리, 규제 준수, 데이터 무결성에 대한 수요 증가를 원동력으로 꾸준히 진전하고 있습니다. 디지털 및 클라우드 기반 솔루션의 도입 확대 외에도 실시간 문서 추적, 자동화된 보고 및 테스트 시설 간의 협력 강화에 대한 필요성이 성장을 가속하고 있습니다.
| 조사 범위 | |
|---|---|
| 조사 대상 기간 | 2024-2033년 |
| 기준 연도 | 2024년 |
| 예측 기간 | 2025-2030년 |
| 대상 단위 | 금액(10억 달러) |
| 부문 | 제공별, 기능별, 배포별, 치료 영역별, 시험 단계별, 최종 사용자별, 지역별 |
| 대상 지역 | 아시아태평양, 북미, 유럽, 라틴아메리카, 중동 및 아프리카, GCC 국가 |
효율성의 이점을 강조하는 Frost & Sullivan(2024년)의 최근 조사는 eTMF 시스템을 통해 감사 및 사이트 준비 시간을 최대 30% 단축할 수 있어 의약품 승인 프로세스를 가속할 수 있다고 추정되고 있습니다. 또한, 제약 및 생명공학 연구에 대한 투자 증가와 세계 임상시험의 복잡성이 결합되어 세계 eTMF 시스템의 도입이 가속화되고 있습니다.

"전자 임상시험 마스터 파일(eTMF) 시스템 시장에서 클라우드 기반 부문은 예측 기간 동안 가장 큰 부문이 됩니다."
도입 형태별로는 확장성·안전성·액세스성이 뛰어난 임상시험 관리 솔루션에 수요 증가를 배경으로, 클라우드 베이스 부문이 전자 임상시험 마스터 파일(eTMF) 시스템 시장에서 최대의 점유율을 차지했습니다. 클라우드 기반 eTMF 시스템은 실시간 문서 액세스, 다중 테스트 사이트 간의 중앙 집중식 협업, 기타 디지털 건강 플랫폼과의 원활한 연동을 가능하게 합니다. 이러한 솔루션은 IT 인프라 비용 절감, 시스템 유지 관리 간소화, 자동 업데이트 및 감사 지원 문서화를 통한 규제 요구 사항을 준수합니다. 임상시험의 복잡화가 진행되고 있는 가운데 원격 모니터링과 효율적인 데이터 관리의 필요성이 높아짐에 따라 제약기업, 생명공학기업, 계약연구기관(CRO)에 클라우드 기반 eTMF 시스템의 세계 도입이 진행되고 있습니다.
2024년 최종 사용자별로 예측 기간 동안 전자 임상시험 마스터 파일(eTMF) 시스템 시장에서 계약연구기관(CRO) 부문이 가장 빠른 성장을 기록했습니다. 외부 위탁되는 임상시험 증가와 연구 프로토콜의 복잡화가 진행되고 있는 가운데, CRO는 효율적이고 컴플라이언스에 준거한 문서 관리를 위해 eTMF 시스템의 도입을 추진하고 있습니다. 이 시스템은 테스트 문서의 실시간 추적을 가능하게 하고, 워크플로우를 간소화하고, 여러 연구 기지 간의 협력을 강화합니다. 또한 eTMF 솔루션이 제공하는 데이터 무결성 강화, 감사 대응 준비 및 규정 준수의 이점도 CRO에게 유용합니다. 시험의 신속한 이행과 비용 효율적인 운영에 대한 수요 증가는 세계 CRO에 의한 eTMF 시스템의 급속한 도입을 더욱 촉진하고 있습니다.
아시아태평양은 예측 기간 동안 전자 임상시험 마스터 파일(eTMF) 시스템 시장에서 가장 높은 성장률을 나타낼 것으로 예측됩니다. 이 지역은 제약 및 생명 공학 분야의 급속한 확대에 견인됩니다. 임상시험활동 증가, CRO(계약연구기관)에의 시험 위탁 확대, 효율적인 시험관리를 위한 디지털 솔루션 도입의 진전이 시장 성장을 가속하는 주요인이 되고 있습니다. 또한 의료 인프라 개선, 정부의 적극적인 시책, 임상 연구 투자 증가 등 첨단 eTMF 시스템 도입을 지원하고 있습니다. 컴플라이언스 확보, 워크플로우 효율화, 데이터 무결성 강화를 위해 클라우드 기반 플랫폼, 분석 및 보고 기능의 도입이 확대되고 있습니다. 이러한 요인들이 함께 아시아태평양은 세계에서 가장 성장이 빠른 지역 시장으로서의 지위를 확립하고 있습니다.
본 보고서에서는 세계의 전자 임상시험 마스터 파일(eTMF) 시스템 시장에 대해 조사했으며 제공별, 기능별, 배포별, 치료영역별, 시험단계별, 최종 사용자별, 지역별 동향, 시장 진출기업 프로파일 등을 정리했습니다.
자주 묻는 질문
목차
제1장 서론
제2장 조사 방법
제3장 주요 요약
제4장 중요 인사이트
제5장 시장 개요
- 서론
- 시장 역학
- 고객의 비즈니스에 영향을 미치는 동향/혼란
- 업계 동향
- 규제 상황
- 가격 분석
- 밸류체인 분석
- 생태계 분석
- 투자 및 자금조달 시나리오
- 기술 분석
- 특허 분석
- 주된 회의와 이벤트(2025-2026년)
- 사례 연구 분석
- Porter's Five Forces 분석
- 주요 이해관계자와 구매 기준
- 미충족 수요(Unmet Needs) 분석
- 비즈니스 모델
- AI/생성형 AI가 전자 임상시험 마스터 파일(eTMF) 시스템 시장에 미치는 영향
- 미국 관세가 전자 임상시험 마스터 파일(eTMF) 시스템 시장에 미치는 영향(2025년)
제6장 전자 임상시험 마스터 파일(eTMF) 시스템 시장, 제공별
- 서론
- 소프트웨어
- 서비스
제7장 전자 임상시험 마스터 파일(eTMF) 시스템, 기능별
- 서론
- 문서 관리
- 워크플로우 및 협업 도구
- 컴플라이언스 및 감사 관리
- 분석 및 보고
- 기타 임상 시스템과의 통합
제8장 전자 임상시험 마스터 파일(eTMF) 시스템 시장, 배포별
- 서론
- 클라우드 기반
- On-Premise
제9장 전자 임상시험 마스터 파일(eTMF) 시스템 시장, 치료 영역별
- 서론
- 종양학
- 심혈관 질환
- 호흡기 질환
- 위장관 장애
- 신경계 장애
- 기타
제10장 전자 임상시험 마스터 파일(eTMF) 시스템 시장, 시험 단계별
- 서론
- PHASE I
- PHASE II
- PHASE III
- PHASE IV
제11장 전자 임상시험 마스터 파일(eTMF) 시스템 시장, 최종 사용자별
- 서론
- 제약 및 바이오테크놀러지 기업
- 의료기기 기업
- CRO
- 학술 및 의료 연구기관
- 정부기관 및 비영리단체
제12장 전자 임상시험 마스터 파일(eTMF) 시스템 시장, 지역별
- 서론
- 북미
- 북미의 거시경제 전망
- 미국
- 캐나다
- 유럽
- 유럽의 거시경제 전망
- 독일
- 프랑스
- 영국
- 이탈리아
- 스페인
- 기타
- 아시아태평양
- 아시아태평양의 거시경제 전망
- 중국
- 일본
- 인도
- 한국
- 호주
- 기타
- 라틴아메리카
- 라틴아메리카의 거시경제 전망
- 브라질
- 멕시코
- 기타
- 중동 및 아프리카
- 중동 및 아프리카의 거시경제 전망
- GCC 국가
- 기타
제13장 경쟁 구도
- 개요
- 주요 진입기업의 전략/강점
- 수익 점유율 분석(2020-2024년)
- 시장 점유율 분석(2024년)
- 브랜드/소프트웨어 비교
- 기업평가와 재무지표
- 기업평가 매트릭스 : 주요 진출기업
- 기업평가 매트릭스 : 스타트업/중소기업(2024년)
- 경쟁 시나리오
제14장 기업 프로파일
- 주요 진출기업
- VEEVA SYSTEMS
- ORACLE
- IQVIA
- MEDIDATA(DASSAULT SYSTEMES)
- TRANSPERFECT
- PHLEXGLOBAL(CENCORA, INC.)
- ARISGLOBAL
- MASTERCONTROL SOLUTIONS, INC.
- ENNOV
- MONTRIUM INC.
- EXTEDO
- SURECLINICAL INC.
- FLORENCE HEALTHCARE
- EGNYTE, INC.
- CLOUDBYZ
- MYCLIN CLINICAL RESEARCH LLC
- OCTALSOFT
- CRUCIAL DATA SOLUTIONS
- DATARIVER SRL
- AUREA, INC.
- 기타 기업
- AGATHA INC.
- EVIDENTIQ
- CLINION
- ANJU SOFTWARE
- CLINEVO TECHNOLOGIES
제15장 부록
KTH 25.11.28The global eTMF systems market is projected to reach USD 2.49 billion by 2030, up from USD 1.36 billion in 2025, at a high CAGR of 12.8% during the forecast period. The market is progressing steadily, driven by the growing demand for streamlined clinical trial management, regulatory compliance, and data integrity. The increasing adoption of digital and cloud-based solutions, coupled with the need for real-time document tracking, automated reporting, and enhanced collaboration across study sites, is fueling growth.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2033 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | Offering, Functionality, Deployment, Therapeutic Area, Trial Phase, End User, and Region |
| Regions covered | Asia Pacific, North America, Europe, Latin America, the Middle East & Africa, and GCC Countries |
Highlighting efficiency benefits, a recent Frost & Sullivan (2024) study estimates that eTMF systems can reduce audit and site preparation time by up to 30%, accelerating drug approval processes. Moreover, rising investments in pharmaceutical and biotechnology research, along with the complexity of global clinical trials, are accelerating the adoption of eTMF systems worldwide.
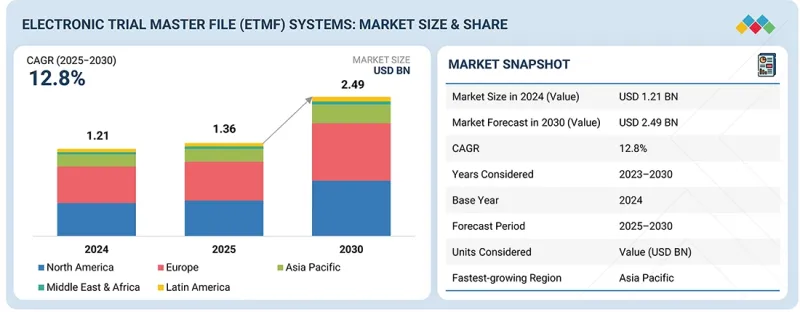
"The cloud-based segment, under the eTMF systems market, is the largest segment during the forecast period."
Based on deployment, the cloud-based segment accounted for the largest share of the eTMF systems market, driven by the growing preference for scalable, secure, and accessible clinical trial management solutions. Cloud-based eTMF systems enable real-time document access, centralized collaboration across multiple study sites, and seamless integration with other digital health platforms. These solutions reduce IT infrastructure costs, simplify system maintenance, and ensure compliance with regulatory requirements through automated updates and audit-ready documentation. The increasing complexity of clinical trials, coupled with the need for remote monitoring and efficient data management, is driving the global adoption of cloud-based eTMF systems among pharmaceutical, biotechnology, and contract research organizations.
"Based on the end user, the contract research organizations (CROs) segment is expected to register the fastest growth during the forecast period."
In 2024, based on end user, the contract research organizations (CROs) segment is expected to register the fastest growth in the eTMF systems market during the forecast period. The rising number of outsourced clinical trials and increasing complexity of study protocols are driving CROs to adopt eTMF systems for efficient and compliant document management. These systems enable the real-time tracking of trial documents, streamline workflows, and enhance collaboration across multiple study sites. Moreover, CROs benefit from enhanced data integrity, audit readiness, and regulatory compliance offered by eTMF solutions. Growing demand for faster trial execution and cost-effective operations is further fueling the rapid adoption of eTMF systems by CROs globally.
"Asia Pacific to witness the highest growth rate during the forecast period."
Asia Pacific is expected to witness the highest growth rate in the eTMF systems market during the forecast period, driven by the rapid expansion of the pharmaceutical and biotechnology sectors across the region. Increasing clinical trial activities, rising outsourcing of trials to Contract Research Organizations (CROs), and growing adoption of digital solutions for efficient trial management are key factors fueling market growth. Moreover, improvements in healthcare infrastructure, favorable government initiatives, and rising investment in clinical research are supporting the adoption of advanced eTMF systems. Cloud-based platforms, analytics, and reporting functionalities are being increasingly implemented to ensure compliance, streamline workflows, and enhance data integrity. The combination of these factors positions the Asia Pacific as the fastest-growing regional market globally.
In-depth interviews have been conducted with chief executive officers (CEOs), Directors, and other executives from various key organizations operating in the authentication and brand protection marketplace. The breakdown of primary participants is as mentioned below:
- By Company Type - Tier 1: 48%, Tier 2: 36%, and Tier 3: 16%
- By Designation - Directors: 15%, Managers: 10%, and Others: 75%
- By Region - North America: 39%, Europe: 31%, Asia Pacific: 21, Latin America: 6%, Middle East & Africa: 3%
Key Players in the Market
The key players operating in the eTMF systems market include Veeva Systems (US), Oracle (US), IQVIA (US), Medidata (Dassault Systemes) (US), TransPerfect (US), Phlexglobal (Cencora, Inc.) (UK), ArisGlobal (US), MasterControl Solutions, Inc. (US), Ennov (UK), Montrium Inc. (Canada), SureClinical Inc. (US), Florence Healthcare (US), Egnyte, Inc. (US), Cloudbyz (US), myClin Clinial Research LLC. (US), Octalsoft (US), Crucial Data Solutions (US), DataRiver S.r.l. (Italy), EXTEDO (Bertelsmann SE & Co. KGaA) (Germany), Aurea, Inc. (US), Agatha Inc. (Japan), EvidentIQ (Germany), Clinion (US), Anju Software Inc. (US), Clinevo Technologies (US).
Research Coverage:
The report analyzes the eTMF systems market, aiming to estimate the market size and future growth potential of various market segments based on offering, functionality, deployment, therapeutic area, trial phase, end user, and region. The report also provides a competitive analysis of the key players operating in this market, along with their company profiles, product offerings, recent developments, and key market strategies.
Reasons to Buy the Report
This report will benefit established firms as well as new entrants and smaller firms in gauging the market pulse, which in turn will help them capture a greater share of the market. Firms purchasing the report can use one or a combination of the following strategies to strengthen their market positions.
This report provides insights into:
- Analysis of key drivers (rising number of clinical trials, regulatory compliance and inspection readiness, real-time data management and analysis, support for decentralized and global trials, adoption of cloud-based and automated solutions), restraints (high setup and licensing costs, resistance to change from traditional paper-based systems), opportunities (AI-driven automation & risk-based document management, predictive analytics for compliance & risk management), and challenges (data security & cybersecurity risks, interoperability issues with legacy systems) influencing the growth of the eTMF systems market
- Product Development/Innovation: Detailed insights on upcoming technologies, research & development activities, and new product & service launches in the eTMF systems market
- Market Development: Comprehensive information on the lucrative emerging markets, offering, functionality, deployment, therapeutic area, trial phase, end user, and region
- Market Diversification: Exhaustive information about the product portfolios, growing geographies, recent developments, and investments in the eTMF systems market
- Competitive Assessment: In-depth assessment of market shares, growth strategies, product offerings, and capabilities of the leading players in the eTMF systems market, like Veeva Systems (US), Oracle (US), IQVIA (US), Medidata (US), and Transperfect (US)
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 MARKET SCOPE
- 1.3.1 MARKET SEGMENTATION AND REGIONAL SCOPE
- 1.3.2 INCLUSIONS AND EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.4 CURRENCY CONSIDERED
- 1.5 STAKEHOLDERS
- 1.6 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH APPROACH
- 2.1.1 SECONDARY RESEARCH
- 2.1.1.1 Key data from secondary sources
- 2.1.2 PRIMARY RESEARCH
- 2.1.2.1 Primary sources
- 2.1.2.2 Key data from primary sources
- 2.1.2.3 Breakdown of primaries
- 2.1.2.4 Insights from primary experts
- 2.1.1 SECONDARY RESEARCH
- 2.2 RESEARCH METHODOLOGY DESIGN
- 2.3 MARKET SIZE ESTIMATION
- 2.4 MARKET BREAKDOWN AND DATA TRIANGULATION
- 2.5 MARKET SHARE ESTIMATION
- 2.6 RESEARCH ASSUMPTIONS
- 2.7 RESEARCH LIMITATIONS
- 2.8 RISK ASSESSMENT
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 ATTRACTIVE OPPORTUNITIES FOR PLAYERS IN ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET
- 4.2 NORTH AMERICA: ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY OFFERING AND REGION
- 4.3 ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- 4.4 REGIONAL MIX: ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET (2023-2030)
- 4.5 ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET: DEVELOPED VS. EMERGING ECONOMIES, 2025 VS. 2030 (USD MILLION)
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Rising number of clinical trials
- 5.2.1.2 Growing regulatory scrutiny and need for inspection readiness
- 5.2.1.3 Increasing complexity of clinical trials
- 5.2.1.4 Growing shift toward decentralized and global clinical trial methodologies
- 5.2.1.5 Increasing adoption of cloud-based and automated solutions
- 5.2.2 RESTRAINTS
- 5.2.2.1 High setup and licensing costs
- 5.2.2.2 Resistance to change from traditional paper-based systems
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Integration of artificial intelligence and risk-based approaches
- 5.2.3.2 Predictive analytics for compliance & risk management
- 5.2.4 CHALLENGES
- 5.2.4.1 Data security & cybersecurity risks
- 5.2.4.2 Interoperability challenges with legacy systems
- 5.2.1 DRIVERS
- 5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESS
- 5.4 INDUSTRY TRENDS
- 5.4.1 SHIFT FROM PAPER-BASED TO DIGITAL TRIAL DOCUMENTATION
- 5.4.2 ADOPTION OF CLOUD-BASED AND SCALABLE SOLUTIONS
- 5.4.3 INTEGRATION WITH OTHER CLINICAL SYSTEMS
- 5.4.4 USE OF ARTIFICIAL INTELLIGENCE AND AUTOMATION
- 5.4.5 FOCUS ON REGULATORY COMPLIANCE AND INSPECTION READINESS
- 5.5 REGULATORY LANDSCAPE
- 5.5.1 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.5.2 REGULATORY ANALYSIS
- 5.5.2.1 North America
- 5.5.2.2 Europe
- 5.5.2.3 Asia Pacific
- 5.5.2.4 Latin America
- 5.5.2.5 Middle East & Africa
- 5.6 PRICING ANALYSIS
- 5.6.1 INDICATIVE PRICING ANALYSIS, BY KEY PLAYER
- 5.6.2 INDICATIVE PRICING ANALYSIS, BY REGION
- 5.6.3 PRICING MODELS
- 5.7 VALUE CHAIN ANALYSIS
- 5.7.1 PRODUCT DESIGN & DEVELOPMENT
- 5.7.2 SYSTEM INTEGRATION & WORKFLOW CUSTOMIZATION
- 5.7.3 IMPLEMENTATION
- 5.7.4 COMPLIANCE MONITORING & AUDIT READINESS
- 5.7.5 POST-SALES SERVICES
- 5.8 ECOSYSTEM ANALYSIS
- 5.9 INVESTMENT AND FUNDING SCENARIO
- 5.10 TECHNOLOGY ANALYSIS
- 5.10.1 KEY TECHNOLOGIES
- 5.10.1.1 Document Management Systems (DMS)
- 5.10.1.2 Workflow automation and collaboration tools
- 5.10.1.3 Compliance and regulatory tools
- 5.10.1.4 Cloud storage and security
- 5.10.2 COMPLEMENTARY TECHNOLOGIES
- 5.10.2.1 Artificial Intelligence (AI)/Machine Learning (ML)
- 5.10.2.2 Blockchain
- 5.10.2.3 Robotic Process Automation (RPA)
- 5.10.2.4 Collaboration and communication tools
- 5.10.2.5 Mobile applications
- 5.10.3 ADJACENT TECHNOLOGIES
- 5.10.3.1 CLINICAL TRIAL MANAGEMENT SYSTEMS (CTMS)
- 5.10.3.2 RANDOMIZATION & TRIAL SUPPLY MANAGEMENT (RTSM/IWRS)
- 5.10.3.3 ELECTRONIC DATA CAPTURE (EDC)
- 5.10.3.4 ECONSENT PLATFORMS
- 5.10.3.5 ESOURCE/REMOTE SOURCE DATA CAPTURE
- 5.10.1 KEY TECHNOLOGIES
- 5.11 PATENT ANALYSIS
- 5.12 KEY CONFERENCES AND EVENTS, 2025-2026
- 5.13 CASE STUDY ANALYSIS
- 5.13.1 CASE STUDY 1: ENHANCED CLINICAL EFFICIENCY AND INSPECTION READINESS THROUGH VEEVA ETMF AND CTMS
- 5.13.2 CASE STUDY 2: STREAMLINED CLINICAL TRIAL OPERATIONS WITH ORACLE SIEBEL CTMS
- 5.13.3 CASE STUDY 3: TRANSITION TOWARD ELECTRONIC TMF FOR INSPECTION READINESS
- 5.14 PORTER'S FIVE FORCES ANALYSIS
- 5.14.1 BARGAINING POWER OF SUPPLIERS
- 5.14.2 BARGAINING POWER OF BUYERS
- 5.14.3 THREAT OF NEW ENTRANTS
- 5.14.4 THREAT OF SUBSTITUTES
- 5.14.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.15 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.15.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.15.2 BUYING CRITERIA
- 5.16 UNMET NEEDS ANALYSIS
- 5.16.1 CURRENT UNMET NEEDS
- 5.16.2 END-USER EXPECTATIONS
- 5.17 BUSINESS MODELS
- 5.17.1 SUBSCRIPTION-BASED (SAAS) MODEL
- 5.17.2 LICENSING MODEL
- 5.17.3 PAY-PER-USE MODEL
- 5.17.4 FREEMIUM MODEL
- 5.17.5 HYBRID MODEL
- 5.18 IMPACT OF AI/GEN AI ON ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET
- 5.18.1 INTRODUCTION
- 5.18.2 MARKET POTENTIAL OF AI/GEN AI ON ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET
- 5.18.3 CASE STUDY RELATED TO AI/GEN AI IMPLEMENTATION
- 5.18.3.1 AI-powered eTMF automation for CRO document processing
- 5.18.4 IMPACT OF AI/GEN AI ON INTERCONNECTED AND ADJACENT ECOSYSTEMS
- 5.18.4.1 Clinical trial services
- 5.18.4.2 eClinical solutions
- 5.18.4.3 AI in clinical trials
- 5.18.5 USER READINESS AND IMPACT ASSESSMENT
- 5.18.5.1 User readiness
- 5.18.5.1.1 User A: Pharmaceutical & Biotechnology Companies
- 5.18.5.1.2 User B: Contract Research Organizations (CROs)
- 5.18.5.2 Impact assessment
- 5.18.5.2.1 User A: Pharmaceutical & Biotechnology Companies
- 5.18.5.2.2 User B: Contract Research Organizations (CROs)
- 5.18.5.1 User readiness
- 5.19 IMPACT OF 2025 US TARIFFS ON ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET
- 5.19.1 INTRODUCTION
- 5.19.2 KEY TARIFF RATES
- 5.19.3 PRICE IMPACT ANALYSIS
- 5.19.4 IMPACT ON COUNTRY/REGION
- 5.19.4.1 US
- 5.19.4.2 Europe
- 5.19.4.3 Asia Pacific
- 5.19.5 IMPACT ON END-USE INDUSTRIES
- 5.19.5.1 Pharmaceutical & biotechnology companies
- 5.19.5.2 Contract Research Organizations (CROs)
6 ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY OFFERING
- 6.1 INTRODUCTION
- 6.2 SOFTWARE
- 6.2.1 STANDALONE ETMF SOFTWARE
- 6.2.1.1 Easy scalability, quick implementation timelines, and reduced IT infrastructure requirements to drive market
- 6.2.2 INTEGRATED ETMF SOFTWARE
- 6.2.2.1 Shift toward unified eClinical ecosystems, cloud-based platforms, and advanced analytics capabilities to propel market
- 6.2.1 STANDALONE ETMF SOFTWARE
- 6.3 SERVICES
- 6.3.1 RISING COMPLEXITY OF GLOBAL AND DECENTRALIZED TRIALS TO EXPEDITE GROWTH
7 ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS, BY FUNCTIONALITY
- 7.1 INTRODUCTION
- 7.2 DOCUMENT MANAGEMENT
- 7.2.1 NEED FOR ADVANCED DOCUMENT MANAGEMENT AND ENHANCED OPERATIONAL TRANSPARENCY TO BOLSTER GROWTH
- 7.3 WORKFLOW & COLLABORATION TOOLS
- 7.3.1 ABILITY TO OFFER STRUCTURED, LARGE-SCALE INFORMATION ON HEALTHCARE SERVICES, UTILIZATION PATTERNS, AND ASSOCIATED COSTS TO BOOST MARKET
- 7.4 COMPLIANCE & AUDIT MANAGEMENT
- 7.4.1 EXPANDING COMPLEXITY OF MULTI-SITE AND HYBRID CLINICAL TRIALS TO CONTRIBUTE TO GROWTH
- 7.5 ANALYTICS & REPORTING
- 7.5.1 INCREASING SHIFT TOWARD DATA-DRIVEN QUALITY MANAGEMENT TO STIMULATE GROWTH
- 7.6 INTEGRATION WITH OTHER ECLINICAL SYSTEMS
- 7.6.1 GROWING ADOPTION OF CLOUD-BASED AND API-ENABLED PLATFORMS TO PROPEL MARKET
8 ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY DEPLOYMENT
- 8.1 INTRODUCTION
- 8.2 CLOUD-BASED DEPLOYMENT
- 8.2.1 RISE OF DECENTRALIZED AND HYBRID CLINICAL TRIAL MODELS TO PROMOTE GROWTH
- 8.3 ON-PREMISES DEPLOYMENT
- 8.3.1 NEED FOR HIGHLY CUSTOMIZED CONFIGURATIONS AND INTEGRATION WITH EXISTING LEGACY SYSTEMS TO AID GROWTH
9 ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY THERAPEUTIC AREA
- 9.1 INTRODUCTION
- 9.2 ONCOLOGY
- 9.2.1 INCREASING SHIFT TOWARD DECENTRALIZED AND HYBRID ONCOLOGY TRIALS TO FACILITATE GROWTH
- 9.3 CARDIOVASCULAR DISEASES
- 9.3.1 SURGE IN TRIAL ACTIVITY TO CONTRIBUTE TO GROWTH
- 9.4 RESPIRATORY DISEASES
- 9.4.1 GROWING EMPHASIS ON DEVELOPING NOVEL BIOLOGICS, INHALATION THERAPIES, VACCINES, AND PRECISION MEDICINE APPROACHES TO BOOST MARKET
- 9.5 GASTROINTESTINAL DISORDERS
- 9.5.1 RISING GLOBAL BURDEN OF GASTROINTESTINAL DISORDERS TO AUGMENT GROWTH
- 9.6 NEUROLOGICAL DISORDERS
- 9.6.1 RISE IN NEUROLOGICAL RESEARCH ACTIVITY TO STIMULATE GROWTH
- 9.7 OTHER THERAPEUTIC AREAS
10 ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY TRIAL PHASE
- 10.1 INTRODUCTION
- 10.2 PHASE I
- 10.2.1 GROWING EARLY-STAGE OUTSOURCING TO DRIVE MARKET
- 10.3 PHASE II
- 10.3.1 INCREASED STUDY ENDPOINTS AND COMPLEX PROTOCOL REQUIREMENTS TO EXPEDITE GROWTH
- 10.4 PHASE III
- 10.4.1 NEED TO ENSURE GLOBAL COMPLIANCE AND MAINTAIN END-TO-END DOCUMENT LIFECYCLE TO BOOST MARKET
- 10.5 PHASE IV
- 10.5.1 INCREASING EMPHASIS ON REAL-WORLD EVIDENCE GENERATION AND PHARMACOVIGILANCE TO SUPPORT GROWTH
11 ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY END USER
- 11.1 INTRODUCTION
- 11.2 PHARMACEUTICAL & BIOTECH COMPANIES
- 11.2.1 RISING REGULATORY SCRUTINY TO ENCOURAGE GROWTH
- 11.3 MEDICAL DEVICE COMPANIES
- 11.3.1 GROWING EMPHASIS ON IMPROVED PATIENT SAFETY OUTCOMES AND AUTOMATED QUALITY CHECKS TO BOOST MARKET
- 11.4 CONTRACT RESEARCH ORGANIZATIONS (CROS)
- 11.4.1 INCREASING FINANCIAL PRESSURE TO CONTROL RISING HEALTHCARE COSTS AND IMPROVE RETURN ON INVESTMENT TO SPUR GROWTH
- 11.5 ACADEMIC & HEALTHCARE RESEARCH INSTITUTIONS
- 11.5.1 NEED FOR DATA INTEGRITY, ACCURATE AUDIT TRAILS, AND CONSISTENT DOCUMENTATION PRACTICES TO FUEL MARKET
- 11.6 GOVERNMENT & NON-PROFIT ORGANIZATIONS
- 11.6.1 INCREASING EMPHASIS ON REGULATORY COMPLIANCE, DATA TRANSPARENCY, AND EFFICIENT RESOURCE UTILIZATION TO DRIVE MARKET
12 ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET, BY REGION
- 12.1 INTRODUCTION
- 12.2 NORTH AMERICA
- 12.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA
- 12.2.2 US
- 12.2.2.1 Robust clinical research ecosystem and regulatory frameworks to aid growth
- 12.2.3 CANADA
- 12.2.3.1 Growing emphasis on compliance and digital clinical operations to drive market
- 12.3 EUROPE
- 12.3.1 MACROECONOMIC OUTLOOK FOR EUROPE
- 12.3.2 GERMANY
- 12.3.2.1 Increasing transition toward digital documentation and regulatory harmonization to fuel market
- 12.3.3 FRANCE
- 12.3.3.1 Expanding clinical research infrastructure to contribute to growth
- 12.3.4 UK
- 12.3.4.1 Ongoing regulatory transition to expedite growth
- 12.3.5 ITALY
- 12.3.5.1 Fragmented digital infrastructure to limit growth
- 12.3.6 SPAIN
- 12.3.6.1 Growing clinical trial volume and continued investment in digital research infrastructure to boost market
- 12.3.7 REST OF EUROPE
- 12.4 ASIA PACIFIC
- 12.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
- 12.4.2 CHINA
- 12.4.2.1 Growing shift toward global compliance frameworks to fuel market
- 12.4.3 JAPAN
- 12.4.3.1 Rising demand for eTMF systems amid digital trial transformation to boost market
- 12.4.4 INDIA
- 12.4.4.1 Rising number of eTMF-management certification programs to promote growth
- 12.4.5 SOUTH KOREA
- 12.4.5.1 Continued support for biopharmaceutical innovations to drive market
- 12.4.6 AUSTRALIA
- 12.4.6.1 Increased digital health and clinical research modernization initiatives to bolster growth
- 12.4.7 REST OF ASIA PACIFIC
- 12.5 LATIN AMERICA
- 12.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA
- 12.5.2 BRAZIL
- 12.5.2.1 Growing participation in global multicenter clinical trials to fuel market
- 12.5.3 MEXICO
- 12.5.3.1 Growing number of research-active hospitals to fuel market
- 12.5.4 REST OF LATIN AMERICA
- 12.6 MIDDLE EAST & AFRICA
- 12.6.1 MACROECONOMIC OUTLOOK FOR MIDDLE EAST & AFRICA
- 12.6.2 GCC COUNTRIES
- 12.6.2.1 Saudi Arabia
- 12.6.2.1.1 Growing emphasis on transparency, quality assurance, and digital oversight to propel market
- 12.6.2.2 UAE
- 12.6.2.2.1 Favorable digital health transformation to aid growth
- 12.6.2.3 Rest of GCC countries
- 12.6.2.1 Saudi Arabia
- 12.6.3 REST OF MIDDLE EAST & AFRICA
13 COMPETITIVE LANDSCAPE
- 13.1 OVERVIEW
- 13.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 13.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN ELECTRONIC TRIAL MASTER FILE (ETMF) SYSTEMS MARKET
- 13.3 REVENUE SHARE ANALYSIS, 2020-2024
- 13.4 MARKET SHARE ANALYSIS, 2024
- 13.5 BRAND/SOFTWARE COMPARISON
- 13.6 COMPANY VALUATION AND FINANCIAL METRICS
- 13.6.1 COMPANY VALUATION
- 13.6.2 FINANCIAL METRICS
- 13.7 COMPANY EVALUATION MATRIX: KEY PLAYERS
- 13.7.1 STARS
- 13.7.2 EMERGING LEADERS
- 13.7.3 PERVASIVE PLAYERS
- 13.7.4 PARTICIPANTS
- 13.7.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 13.7.5.1 Company footprint
- 13.7.5.2 Region footprint
- 13.7.5.3 Offering footprint
- 13.7.5.4 Functionality footprint
- 13.7.5.5 End-user footprint
- 13.8 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 13.8.1 PROGRESSIVE COMPANIES
- 13.8.2 RESPONSIVE COMPANIES
- 13.8.3 DYNAMIC COMPANIES
- 13.8.4 STARTING BLOCKS
- 13.8.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 13.8.5.1 Detailed list of key startups/SMEs
- 13.8.5.2 Competitive benchmarking of startups/SMEs
- 13.9 COMPETITIVE SCENARIO
- 13.9.1 PRODUCT LAUNCHES AND ENHANCEMENTS
- 13.9.2 DEALS
- 13.9.3 OTHER DEVELOPMENTS
14 COMPANY PROFILES
- 14.1 KEY PLAYERS
- 14.1.1 VEEVA SYSTEMS
- 14.1.1.1 Business overview
- 14.1.1.2 Products offered
- 14.1.1.3 Recent developments
- 14.1.1.3.1 Product launches and enhancements
- 14.1.1.3.2 Deals
- 14.1.1.4 MnM view
- 14.1.1.4.1 Right to win
- 14.1.1.4.2 Strategic choices
- 14.1.1.4.3 Weaknesses and competitive threats
- 14.1.2 ORACLE
- 14.1.2.1 Business overview
- 14.1.2.2 Products offered
- 14.1.2.3 Recent developments
- 14.1.2.3.1 Product launches and enhancements
- 14.1.2.3.2 Deals
- 14.1.2.4 MnM view
- 14.1.2.4.1 Right to win
- 14.1.2.4.2 Strategic choices
- 14.1.2.4.3 Weaknesses and competitive threats
- 14.1.3 IQVIA
- 14.1.3.1 Business overview
- 14.1.3.2 Products offered
- 14.1.3.3 Recent developments
- 14.1.3.3.1 Product launches and enhancements
- 14.1.3.3.2 Deals
- 14.1.3.4 MnM view
- 14.1.3.4.1 Right to win
- 14.1.3.4.2 Strategic choices
- 14.1.3.4.3 Weaknesses and competitive threats
- 14.1.4 MEDIDATA (DASSAULT SYSTEMES)
- 14.1.4.1 Business overview
- 14.1.4.2 Products offered
- 14.1.4.3 Recent developments
- 14.1.4.3.1 Product launches and enhancements
- 14.1.4.3.2 Deals
- 14.1.4.4 MnM view
- 14.1.4.4.1 Right to win
- 14.1.4.4.2 Strategic choices
- 14.1.4.4.3 Weaknesses and competitive threats
- 14.1.5 TRANSPERFECT
- 14.1.5.1 Business overview
- 14.1.5.2 Products offered
- 14.1.5.3 Recent developments
- 14.1.5.3.1 Product launches and enhancements
- 14.1.5.3.2 Deals
- 14.1.5.4 MnM view
- 14.1.5.4.1 Right to win
- 14.1.5.4.2 Strategic choices
- 14.1.5.4.3 Weaknesses and competitive threats
- 14.1.6 PHLEXGLOBAL (CENCORA, INC.)
- 14.1.6.1 Business overview
- 14.1.6.2 Products offered
- 14.1.6.3 Recent developments
- 14.1.6.3.1 Product launches and enhancements
- 14.1.6.3.2 Deals
- 14.1.7 ARISGLOBAL
- 14.1.7.1 Business overview
- 14.1.7.2 Products offered
- 14.1.7.3 Recent developments
- 14.1.7.3.1 Deals
- 14.1.8 MASTERCONTROL SOLUTIONS, INC.
- 14.1.8.1 Business overview
- 14.1.8.2 Products offered
- 14.1.8.3 Recent developments
- 14.1.8.3.1 Deals
- 14.1.8.3.2 Other developments
- 14.1.9 ENNOV
- 14.1.9.1 Business overview
- 14.1.9.2 Products offered
- 14.1.9.3 Recent developments
- 14.1.9.3.1 Product launches and enhancements
- 14.1.9.3.2 Deals
- 14.1.10 MONTRIUM INC.
- 14.1.10.1 Business overview
- 14.1.10.2 Products offered
- 14.1.10.3 Recent developments
- 14.1.10.3.1 Product launches and enhancements
- 14.1.10.3.2 Deals
- 14.1.11 EXTEDO
- 14.1.11.1 Business overview
- 14.1.11.2 Products offered
- 14.1.11.3 Recent developments
- 14.1.11.3.1 Product launches and enhancements
- 14.1.11.3.2 Deals
- 14.1.12 SURECLINICAL INC.
- 14.1.12.1 Business overview
- 14.1.12.2 Products offered
- 14.1.12.3 Recent developments
- 14.1.12.3.1 Deals
- 14.1.13 FLORENCE HEALTHCARE
- 14.1.13.1 Business overview
- 14.1.13.2 Products offered
- 14.1.13.3 Recent developments
- 14.1.13.3.1 Product launches and enhancements
- 14.1.13.3.2 Deals
- 14.1.14 EGNYTE, INC.
- 14.1.14.1 Business overview
- 14.1.14.2 Products offered
- 14.1.14.3 Recent developments
- 14.1.14.3.1 Product launches and enhancements
- 14.1.14.3.2 Deals
- 14.1.14.3.3 Other developments
- 14.1.15 CLOUDBYZ
- 14.1.15.1 Business overview
- 14.1.15.2 Products offered
- 14.1.15.3 Recent developments
- 14.1.15.3.1 Deals
- 14.1.16 MYCLIN CLINICAL RESEARCH LLC
- 14.1.16.1 Business overview
- 14.1.16.2 Products offered
- 14.1.17 OCTALSOFT
- 14.1.17.1 Business overview
- 14.1.17.2 Products offered
- 14.1.18 CRUCIAL DATA SOLUTIONS
- 14.1.18.1 Business overview
- 14.1.18.2 Products offered
- 14.1.18.3 Recent developments
- 14.1.18.3.1 Product launches and enhancements
- 14.1.18.3.2 Deals
- 14.1.19 DATARIVER S.R.L.
- 14.1.19.1 Business overview
- 14.1.19.2 Products offered
- 14.1.20 AUREA, INC.
- 14.1.20.1 Business overview
- 14.1.20.2 Products offered
- 14.1.1 VEEVA SYSTEMS
- 14.2 OTHER PLAYERS
- 14.2.1 AGATHA INC.
- 14.2.2 EVIDENTIQ
- 14.2.3 CLINION
- 14.2.4 ANJU SOFTWARE
- 14.2.5 CLINEVO TECHNOLOGIES
15 APPENDIX
- 15.1 DISCUSSION GUIDE
- 15.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 15.3 CUSTOMIZATION OPTIONS
- 15.4 RELATED REPORTS
- 15.5 AUTHOR DETAILS
















