
|
시장보고서
상품코드
1721538
POC 응고 검사 제품 시장 기회, 성장 촉진요인, 산업 동향 분석 및 예측(2025-2034년)Point of Care Coagulation Testing Products Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
세계의 POC 응고 검사 제품 시장 규모는 2024년 28억 달러로 평가되었고, CAGR 6.2%를 나타내 2034년에는 52억 달러에 이를 것으로 예측되고 있습니다. 즉각적이고 신뢰할 수 있는 결과에 대한 신뢰가 높아짐에 따라 이러한 장비는 현재 긴급 상황, 외과적 치료 및 만성 질환의 장기 관리에 필수적입니다. 게다가 의료의 분산화 모델이나 휴대형 진단 기기로의 변화가, 시장의 확대를 더욱 뒷받침하고 있습니다.

시장은 활성화 응고 시간, d 다이머 검사, 혈소판 수, 프로트롬빈 시간 검사, 기타 카테고리 등, 검사의 유형에 의해 구분됩니다. 2025년부터 2034년까지 연평균 6.3%의 성장률을 나타낼 것으로 예상됩니다. 이 부문의 이점은 주로 의사가 항응고제의 용량을 정확하게 조정하는 데 도움이 되는 신속하고 실용적인 데이터를 제공하는 능력 때문입니다. 심각한 합병증으로 이어질 수 있는 환경에서 프로트롬빈 시간 검사가 제공하는 신속하고 신뢰할 수 있는 결과는 특히 중증 환자 및 장기 치료 관리에서 임상 판단에 필수적입니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 28억 달러 |
| 예측 금액 | 52억 달러 |
| CAGR | 6.2% |
제품 유형별로 보면 시장은 기구와 소모품으로 나뉘어 2024년 점유율은 기구가 63%를 차지했습니다. 이 부문은 2034년까지 33억 달러를 창출할 것으로 예측됩니다. 성을 향상시킬 수 있는 기구가 높게 지지되어 있기 때문에 휴대성이 뛰어나 디지털 헬스케어 시스템과의 원활한 통합이 가능하기 때문에 구명 처치를 위해 신속한 응고 프로파일이 불가결한 병원과 외래 모두에서 필수적인 기기가 되고 있습니다.
미국의 POC 응고 검사 제품 시장은 2023년 8억 5,140만 달러를 창출했습니다. 이 지역 시장 성장을 뒷받침하는 것은 만성 혈액 질환의 유병률 증가와 실시간 진단 도구에 대한 수요 증가입니다. 엄격한 규제 프로토콜에도 불구하고 미국 시장이 계속 성장하고 있는 이유는 유리한 상환 모델과 최신 진단 혁신의 도입에 열성적인 의료 인프라가 있기 때문입니다.
Abbott Laboratories, Sysmex Corporation, Thermo Fisher Scientific, Siemens Healthineers, F. Hoffmann-La Roche와 같은 대기업은 이 성장의 최전선에 있습니다. 이러한 기업들은 휴대용, 사용자 친화적인 시스템을 신속한 납기로 개발함으로써 지속적으로 혁신을 계속하고 있습니다. 연구 협력 투자, 신흥 시장 진출, 클라우드 기반 연결성 강화는 시장에서의 존재감을 높이고 전 세계적으로 임상 결과를 개선하는 중요한 전략입니다.
목차
제1장 조사 방법과 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 미치는 영향요인
- 성장 촉진요인
- 만성 혈액 질환의 유병률의 상승
- 혈액 관련 질환을 억제하기 위한 정부 대처의 확대
- POC 검사 제품의 채용 증가
- 기술적 진보
- 업계의 잠재적 위험 및 과제
- 엄격한 규제 틀
- 높은 제품 개발 비용
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 기술적 상황
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 서론
- 기업의 시장 점유율 분석
- 기업 매트릭스 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 전략 대시보드
제5장 시장 추계·예측 : 검사 유형별(2021-2034년)
- 주요 동향
- 프로트롬빈 시간 검사 제품
- 활성 응고 시간(ACT/APTT) 검사 제품
- 혈소판 수
- D 다이머 검사
- 기타 검사 유형
제6장 시장 추계·예측 : 제품 유형별(2021-2034년)
- 주요 동향
- 기기
- 소모품
제7장 시장 추계·예측 : 최종 용도별(2021-2034년)
- 주요 동향
- 병원
- 진단센터
- 재택 케어 환경
- 기타 용도
제8장 시장 추계·예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제9장 기업 프로파일
- Abbott Laboratories
- Alere
- A&T Corporation
- Diagnostica Stago Sas
- F. Hoffmann-La Roche
- Genrui Biotech
- Helena Laboratories
- Horiba
- Medtronic
- Nihon Kohden Corporation
- Micropoint Biosciences
- Maccura Biotechnology
- Sysmex Corporation
- Siemens Healthineers
- Thermo Fisher Scientific
The Global Point of Care Coagulation Testing Products Market was valued at USD 2.8 billion in 2024 and is estimated to grow at a CAGR of 6.2% to reach USD 5.2 billion by 2034. These innovative devices are transforming patient care by providing real-time coagulation assessments directly at the bedside or clinical point of care, eliminating the need for central laboratory testing. With a growing reliance on immediate and reliable results, these devices are now integral in emergency situations, surgical procedures, and long-term management of chronic illnesses. The market's growth is driven by the increasing incidence of cardiovascular diseases and blood disorders, alongside the rising demand for rapid diagnoses and real-time monitoring. As healthcare providers turn to these tools to support critical medical decisions, the demand for coagulation testing products is intensifying globally. Moreover, the shift toward decentralized healthcare models and portable diagnostic devices further fuels the market's expansion. These tools not only optimize workflow but also enhance patient outcomes by enabling timely interventions.
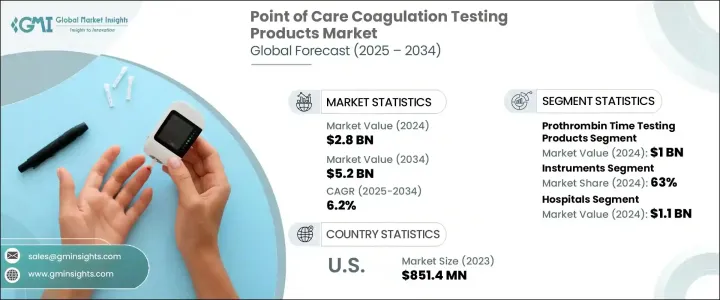
The market is segmented based on test types, including activated clotting time, d-dimer tests, platelet count, prothrombin time testing, and other categories. Among these, the prothrombin time testing segment emerged as a leader, generating USD 1 billion in 2024. It is projected to grow at a CAGR of 6.3% between 2025 and 2034. The segment's dominance is primarily due to its ability to provide fast and actionable data that aids physicians in precisely adjusting anticoagulant dosages. In settings where delays in coagulation monitoring can lead to severe complications, the rapid and dependable results offered by prothrombin time tests have become critical in making clinical decisions, particularly in critical care and long-term therapy management.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2.8 Billion |
| Forecast Value | $5.2 Billion |
| CAGR | 6.2% |
In terms of product type, the market divides into instruments and consumables, with instruments holding a 63% share in 2024. This segment is expected to generate USD 3.3 billion by 2034, as instruments are highly favored for their ability to provide immediate results, thereby improving efficiency in high-pressure clinical environments. Their portability and seamless integration with digital healthcare systems make them indispensable in both hospital and outpatient settings, where quick coagulation profiles are essential for life-saving interventions.
The U.S. Point of Care Coagulation Testing Products Market generated USD 851.4 million in 2023. The market's growth in the region is supported by the rising prevalence of chronic hematologic conditions, coupled with a growing demand for real-time diagnostic tools. Despite stringent regulatory protocols, the U.S. market continues to thrive due to favorable reimbursement models and a healthcare infrastructure that is eager to embrace the latest diagnostic innovations.
Leading players such as Abbott Laboratories, Sysmex Corporation, Thermo Fisher Scientific, Siemens Healthineers, F. Hoffmann-La Roche, and others are at the forefront of this growth. These companies are continuously innovating by developing portable, user-friendly systems with rapid turnaround times. Their investment in research collaborations, expansion into emerging markets, and enhancement of cloud-based connectivity are key strategies to increase market presence and improve clinical outcomes globally.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of chronic blood disorders
- 3.2.1.2 Growing government initiatives to curb blood-related diseases
- 3.2.1.3 Increasing adoption of point of care testing products
- 3.2.1.4 Technological advancements
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory framework
- 3.2.2.2 High cost of product development
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Porter’s analysis
- 3.7 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Prothrombin time testing products
- 5.3 Activated clotting time (ACT/APTT) testing products
- 5.4 Platelet count
- 5.5 D-dimer test
- 5.6 Others test types
Chapter 6 Market Estimates and Forecast, By Product Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Instruments
- 6.3 Consumables
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Diagnostic centers
- 7.4 Home care settings
- 7.5 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Abbott Laboratories
- 9.2 Alere
- 9.3 A&T Corporation
- 9.4 Diagnostica Stago Sas
- 9.5 F. Hoffmann-La Roche
- 9.6 Genrui Biotech
- 9.7 Helena Laboratories
- 9.8 Horiba
- 9.9 Medtronic
- 9.10 Nihon Kohden Corporation
- 9.11 Micropoint Biosciences
- 9.12 Maccura Biotechnology
- 9.13 Sysmex Corporation
- 9.14 Siemens Healthineers
- 9.15 Thermo Fisher Scientific



















