
|
시장보고서
상품코드
1811751
분자진단 시장(-2030년) : 제품 및 서비스, 검사 유형, 샘플, 기술, 용도(감염증, 암)Molecular Diagnostics Market by Product & Service, Test Type, Sample, Technology, and Application (Infectious, Cancer ) - Global Forecast to 2030 |
||||||
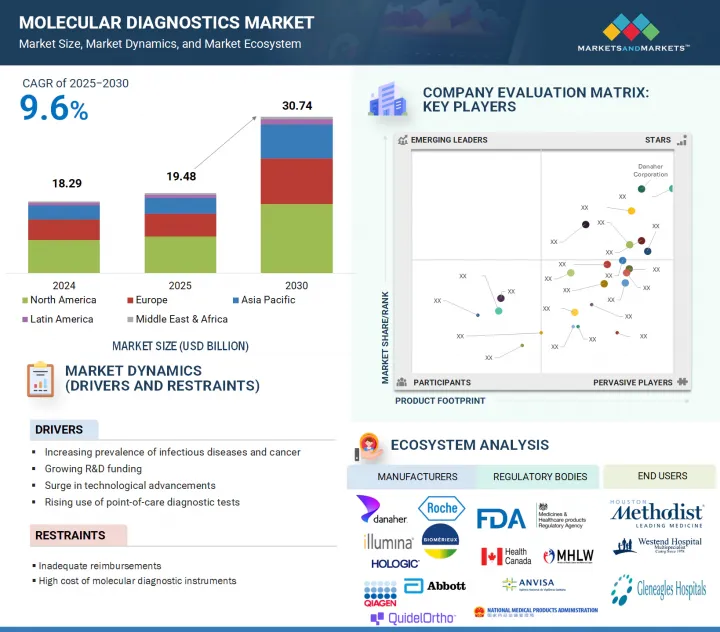
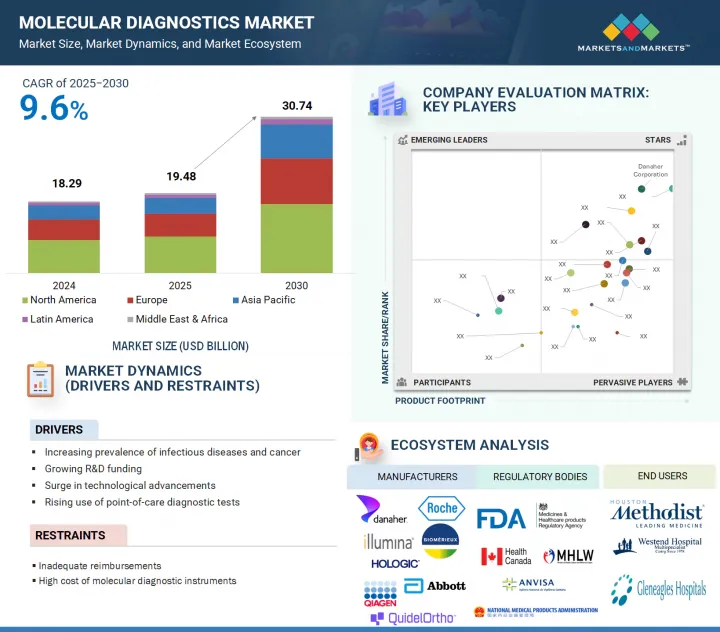
분자진단 시장 규모는 2025년 약 194억 8,000만 달러에서 예측 기간 중 CAGR 9.6%로 성장을 지속하여, 2030년까지 307억 4,000만 달러 규모로 성장할 것으로 예측됩니다.
분자진단은 분자 및 유전자 수준에서 질병을 검출할 수 있기 때문에 현대 의학에서 매우 중요한 역할을 하고 있습니다. 이러한 기술은 높은 민감도와 특이성을 가지고 있어 병원체 및 유전자 이상을 조기에 식별할 수 있습니다. 처리 시간 단축, 자동화 플랫폼, 분석 설계 개선 등 지속적인 기술 혁신을 통해 분자 검사의 신뢰성과 사용 편의성이 크게 향상되었습니다. 이러한 혁신은 다양한 의료 현장의 대규모 검사 시스템을 지원하는 데 있어 매우 중요합니다.
| 조사 범위 | |
|---|---|
| 조사 대상 연도 | 2024-2030년 |
| 기준 연도 | 2024년 |
| 예측 기간 | 2025-2030년 |
| 단위 | 금액(달러) |
| 부문 | 제품 및 서비스, 검사 유형, 샘플 유형, 기술, 용도, 방법, 임상 응용, 최종사용자 |
| 대상 지역 | 북미, 유럽, 아시아태평양, 라틴아메리카, 중동 및 아프리카 |
또한, 인도와 같은 국가에서는 정부 및 민간 부문의 노력으로 국민들의 인식이 높아지고 진단 및 치료 서비스에 대한 접근성이 확대되고 있습니다. 이러한 노력은 향후 몇 년 동안 분자진단의 도입을 가속화할 것으로 예측됩니다.
"샘플 유형별로는 혈액, 혈청, 혈장 부문이 2024년 시장을 지배할 것"
이는 바이러스 감염 및 종양성 질환을 포함한 광범위한 질병을 검출하는 데 있어 이러한 샘플 유형이 높은 민감도와 특이성을 가지고 있기 때문입니다. 또한, 운송 및 보관 시 안정성이 뛰어나 중앙 집중식 실험실 및 현장 진료 환경에 이상적입니다. 또한, 개인 맞춤형 의료의 확대와 임상 의사 결정에 있어 바이오마커의 사용 증가는 혈액 기반 분자 검사에 대한 수요를 증가시키고 있습니다. 이러한 장점으로 인해 이 부문은 앞으로도 견조한 성장세를 이어갈 것으로 예측됩니다.
"기술별로는 DNA 시퀀싱&차세대 시퀀싱(NGS) 부문이 예측 기간 동안 가장 높은 성장세를 보일 것으로 예측됩니다."
이러한 급격한 성장은 유전자 변이 검출, 감염 인자 확인, 개인별 맞춤 치료 계획 수립에 대한 활용 확대에 의해 촉진되고 있습니다. 또한, 처리 속도 향상과 데이터 정확도 향상으로 연구 및 임상 현장에서의 채용이 확대되고 있습니다. 또한, NGS는 높은 처리량 성능과 종합적인 유전체 프로파일링에 대한 유용성으로 주목받고 있습니다. 정밀의료가 발전함에 따라 시퀀싱 기반 진단 수요는 크게 증가할 것으로 예측됩니다.
"아시아태평양이 예측 기간 동안 가장 빠르게 성장하는 지역 시장이 될 것으로 예측됩니다."
이러한 성장은 의료 투자 증가, 질병 조기 발견에 대한 인식 제고, 신흥국에서의 첨단 진단 기술 도입 확대에 힘입은 바 큽니다. 특히 중국, 인도, 한국 등의 국가에서는 의료 인프라와 검사실 역량이 빠르게 개선되고 있습니다. 또한, 감염병 감시 강화와 분자 검사에 대한 접근성 확대를 위한 정부의 이니셔티브도 시장 성장에 기여하고 있습니다. 또한, 대규모 환자 인구와 개인 맞춤형 의료 솔루션에 대한 수요 증가는 이 지역의 성장 잠재력을 더욱 높여주고 있습니다.
세계의 분자진단(Molecular Diagnostics) 시장을 조사했으며, 시장 개요, 시장 성장에 영향을 미치는 각종 영향요인 분석, 기술 및 특허 동향, 법 및 규제 환경, 사례 분석, 시장 규모 추이 및 예측, 각종 부문별/지역별/주요 국가별 상세 분석, 경쟁 구도, 주요 기업 개요 등의 정보를 정리하여 전해드립니다.
목차
제1장 서론
제2장 조사 방법
제3장 주요 요약
제4장 프리미엄 인사이트
제5장 시장 개요
- 시장 역학
- 성장 촉진요인
- 성장 억제요인
- 기회
- 과제
- 가격 분석
- 특허 분석
- 밸류체인 분석
- 공급망 분석
- HS코드
- 생태계 분석
- Porter의 Five Forces 분석
- 규제 상황
- 기술 분석
- 2025-2026년 주요 컨퍼런스 및 이벤트
- 고객 사업에 영향을 미치는 동향/혼란
- 주요 이해관계자와 구입 기준
- 투자 및 자금조달 시나리오
- 사례 연구 분석
- AI/생성형 AI가 분자진단 시장에 미치는 영향
- 미국의 2025년 관세
제6장 분자진단 시장 : 제품 및 서비스별
- 시약 및 키트
- 기기
- 서비스 및 소프트웨어
제7장 분자진단 시장 : 검사 유형별
- 실험실 검사
- POC 검사
제8장 분자진단 시장 : 샘플 유형별
- 혈액, 혈청 및 혈장
- 소변
- 기타
제9장 분자진단 시장 : 기술별
- 중합효소 연쇄반응
- 등온 핵산 증폭 기술
- DNA 시퀀싱 및 차세대 시퀀싱
- IN SITU HYBRIDIZATION
- DNA 마이크로어레이
- 기타
제10장 분자진단 시장 : 용도별
- 감염증 진단
- 성 감염증
- 간염
- 호흡기 감염증
- 원내 감염증
- 매개성 질환
- 기타
- 암 검사
- 유방암
- 대장암
- 폐암
- 전립선암
- 기타
- 유전자 검사
- 기타
제11장 분자진단 시장 : 기술별
- 멀티플렉스 테스트
- 싱글플렉스 테스트
제12장 분자진단 시장 : 임상 응용별
- 진단
- 스크리닝
제13장 분자진단 시장 : 최종사용자별
- 진단실험실
- 병원 및 클리닉
- 기타
제14장 분자진단 시장 : 지역별
- 북미
- 거시경제 전망
- 미국
- 캐나다
- 유럽
- 거시경제 전망
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 러시아
- 스위스
- 기타
- 아시아태평양
- 거시경제 전망
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타
- 라틴아메리카
- 거시경제 전망
- 브라질
- 멕시코
- 기타
- 중동 및 아프리카
- 거시경제 전망
- 사우디아라비아
- 아랍에미리트(UAE)
- 기타
제15장 경쟁 구도
- 주요 시장 진출기업의 전략/강점
- 매출 분배 분석
- 시장 점유율 분석
- 기업 평가 매트릭스 : 주요 기업
- 기업 평가 매트릭스 : 스타트업 및 중소기업
- 기업 평가와 재무 지표
- 브랜드 및 제품 비교
- 경쟁 시나리오
제16장 기업 개요
- 주요 기업
- DANAHER CORPORATION
- F. HOFFMANN-LA ROCHE LTD
- ILLUMINA, INC.
- HOLOGIC, INC.
- BIOMERIEUX
- ABBOTT LABORATORIES
- THERMO FISHER SCIENTIFIC INC.
- QIAGEN N.V.
- REVVITY, INC.
- MYRIAD GENETICS, INC.
- SIEMENS HEALTHINEERS AG
- 기타 기업
- BECTON, DICKINSON AND COMPANY(BD)
- GRIFOLS, S.A.
- QUIDELORTHO CORPORATION
- DIASORIN S.P.A.
- EXACT SCIENCES CORPORATION
- GENETIC SIGNATURES
- AGILENT TECHNOLOGIES, INC.
- MDXHEALTH
- BIOCARTIS
- MEDIGEN BIOTECHNOLOGY CORP.
- VELA DIAGNOSTICS
- AMOY DIAGNOSTICS CO., LTD.
- BRUKER CORPORATION(ELITECHGROUP)
- MOLBIO DIAGNOSTICS LIMITED
- GENEOMBIOTECHNOLOGIES
- SAVYON DIAGNOSTICS
- UNIOGEN OY
제17장 부록
LSH 25.09.23The molecular diagnostics market is valued at an estimated USD 19.48 billion in 2025 and is projected to reach USD 30.74 billion by 2030 at a CAGR of 9.6% during the forecast period. Molecular diagnostics plays a vital role in modern healthcare by enabling disease detection at the molecular and genetic level. These technologies offer high sensitivity and specificity, allowing for the early identification of pathogens and genetic abnormalities. Continuous technological advancements, such as faster processing times, automated platforms, and improved assay designs, have significantly enhanced the reliability and accessibility of molecular tests. These innovations are significant in supporting large-scale testing efforts across diverse healthcare settings.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | Product & service, test type, sample type, technology, application, technique, clinical application, end user |
| Regions covered | North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
Moreover, in countries like India, government and private sector initiatives contribute to increased public awareness and greater access to diagnostic and treatment services. Such efforts are expected to accelerate the adoption of molecular diagnostics in the coming years.
"By sample type, the blood, serum, and plasma segment dominated the molecular diagnostics market in 2024."
By sample type, the molecular diagnostics market is segmented into blood, serum & plasma; urine; and other sample types. The blood, serum, and plasma segment accounted for the largest share of the market in 2024. This is attributed to the high sensitivity and specificity these sample types offer in detecting a wide range of diseases, including viral infections and oncological conditions. Their stability during transportation and storage makes them ideal for centralized laboratories and point-of-care settings. Additionally, the growing adoption of personalized medicine and increased use of biomarkers in clinical decision-making fuel the demand for blood-based molecular testing. These advantages collectively position the segment for continued strong growth.
"By technology, the DNA sequencing & next-generation sequencing (NGS) segment is projected to achieve the highest growth during the forecast period."
By technology, the molecular diagnostics market is bifurcated into polymerase chain reaction (PCR), isothermal nucleic acid amplification technology, in situ hybridization, DNA sequencing & next-generation sequencing (NGS), DNA microarrays, and other technologies. The DNA sequencing & next-generation sequencing (NGS) segment is projected to witness the highest growth during the forecast period. This surge is driven by their expanding use in detecting genetic mutations, identifying infectious agents, and guiding personalized treatment plans. The increasing turnaround time and data accuracy improvements have also broadened their adoption in research and clinical settings. Moreover, NGS is gaining traction due to its high-throughput capabilities and utility in comprehensive genomic profiling. As precision medicine continues to advance, the demand for sequencing-based diagnostics is set to rise significantly.
"Asia Pacific is projected to be the fastest-growing regional market during the forecast period."
The market for molecular diagnostics is categorized into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. Asia Pacific is projected to witness the highest growth in the global molecular diagnostics market during the forecast period. This growth is supported by the rising healthcare investments, growing awareness of early disease detection, and increasing adoption of advanced diagnostic technologies across emerging economies. Countries such as China, India, and South Korea are experiencing rapid improvements in healthcare infrastructure and laboratory capacity. In addition, government initiatives aimed at strengthening infectious disease surveillance and expanding access to molecular testing are contributing to market growth. The large patient population and rising demand for personalized healthcare solutions further reinforce the region's growth potential.
Break-up of primary participant profiles in the molecular diagnostics market:
- By Company Type: Tier 1 - 40%, Tier 2 -30%, and Tier 3 - 30%
- By Designation: C-level - 27%, Director-level - 18%, and Others - 55%
- By Region: North America - 51%, Europe - 21%, Asia Pacific - 18%, Latin America - 6%, and Middle East & Africa - 4%
The key players in the molecular diagnostics market are Danaher Corporation (US), F. Hoffmann-La Roche Ltd. (Switzerland), Hologic, Inc. (US), Illumina, Inc. (US), Abbott Laboratories (US), bioMerieux (France), Thermo Fisher Scientific Inc. (US), QIAGEN N.V. (Netherlands), Revvity, Inc. (US), Myriad Genetics, Inc. (US), Siemens Healthineers AG (Germany), Becton, Dickinson and Company (BD) (US), Grifols, S.A. (Spain), QuidelOrtho Corporation (US), DiaSorin S.p.A. (Italy), Exact Sciences Corporation (US), Genetic Signatures (Australia), Agilent Technologies, Inc. (US), MDxHealth (Belgium), Biocartis (Belgium), Bruker (US), TBG Diagnostics Limited (Australia), Amoy Diagnostics Co., Ltd. (China), Vela Diagnostics (Singapore), Molbio Diagnostics Pvt. Ltd. (India), geneOmbio Technologies (India), Savyon Diagnostics (Israel), and Uniogen OY (Finland).
Research Coverage:
This research report categorizes the molecular diagnostics market by product & service (reagents & kits, instruments, and services), test type (lab tests and PoC tests), sample type (blood, serum & plasma; urine; and other sample types), technology (polymerase chain reaction (PCR), isothermal nucleic acid amplification technology, in situ hybridization, DNA sequencing & next-generation sequencing (NGS), DNA microarrays, and other technologies), application {infectious disease diagnostics [hepatitis (hepatitis B, hepatitis C & other hepatitis diseases), sexually transmitted diseases (HIV, CT/NG, HPV, syphilis, & other sexually transmitted diseases), respiratory infectious diseases (tuberculosis, influenza, pharyngitis, & other respiratory infectious diseases), hospital-acquired infections, vector-borne diseases, and other infectious diseases], oncology testing (breast cancer, colorectal cancer, lung cancer, prostate cancer, and other cancers), genetic testing and other applications}, technique (multiplex testing and singleplex testing), clinical application (diagnostic and screening), end user (hospitals & clinics, diagnostic laboratories, and other end users), and region (North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa).
The scope of the report covers detailed information regarding the major factors, such as drivers, restraints, opportunities, and challenges influencing the growth of the molecular diagnostics market. A thorough analysis of the key industry players provides insights into their business overview, solutions, key strategies, acquisitions, and agreements. This report also covers a competitive analysis of upcoming startups in the molecular diagnostics market ecosystem.
Reasons to buy this report:
The report will help the market leaders/new entrants with information on the closest approximations of the revenue numbers for the overall molecular diagnostics market and the subsegments. It will also help stakeholders understand the competitive landscape and gain more insights to position their businesses better and plan suitable go-to-market strategies. The report will help stakeholders understand the market pulse, providing information on key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
- Analysis of key drivers [Increasing prevalence of infectious diseases and cancer, growing R&D funding, surge in technological advancements in molecular diagnostics, and rising use of point-of-care (PoC) diagnostic tests], opportunities (Growing significance of companion diagnostics and increasing growth opportunities in emerging economies), restraints (Inadequate reimbursements and high cost of molecular diagnostic instruments), and challenges (Changing regulatory landscape, operational barriers and labor shortage, and introduction of alternative technologies) influencing the growth of the molecular diagnostics market.
- Product Development/Innovation: Detailed insights into upcoming technologies, research & development activities, and new product launches in the molecular diagnostics market
- Market Development: Comprehensive information about lucrative markets across varied regions
- Market Diversification: Exhaustive information about products, untapped geographies, recent developments, and investments in the molecular diagnostics market
- Competitive Assessment: In-depth assessment of market share, growth strategies, product offerings of leading players like Danaher (US), F. Hoffmann-La Roche Ltd (Switzerland), Illumina, Inc. (US), bioMerieux (France), and Hologic, Inc. (US)
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION
- 1.3.2 REGIONAL SCOPE
- 1.4 INCLUSIONS & EXCLUSIONS
- 1.5 YEARS CONSIDERED
- 1.6 CURRENCY CONSIDERED
- 1.7 KEY STAKEHOLDERS
- 1.8 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Key secondary sources
- 2.1.1.2 Key data from secondary sources
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Key primary sources
- 2.1.2.2 Key data from primary sources
- 2.1.2.3 Key industry insights
- 2.1.2.4 Breakdown of primary interviews
- 2.1.1 SECONDARY DATA
- 2.2 MARKET SIZE ESTIMATION
- 2.2.1 BOTTOM-UP APPROACH
- 2.2.1.1 Approach 1: Company revenue estimation approach
- 2.2.1.2 Approach 2: Presentation of companies and primary interviews
- 2.2.1.3 Growth forecast
- 2.2.1.4 CAGR projections
- 2.2.2 TOP-DOWN APPROACH
- 2.2.1 BOTTOM-UP APPROACH
- 2.3 MARKET BREAKDOWN AND DATA TRIANGULATION
- 2.4 MARKET SHARE ASSESSMENT
- 2.5 RESEARCH ASSUMPTIONS
- 2.5.1 PARAMETRIC ASSUMPTIONS
- 2.6 RESEARCH LIMITATIONS
- 2.7 RISK ASSESSMENT
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 MOLECULAR DIAGNOSTICS MARKET OVERVIEW
- 4.2 MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2025 VS. 2030
- 4.3 MOLECULAR DIAGNOSTICS MARKET, BY TEST TYPE, 2025 VS. 2030
- 4.4 MOLECULAR DIAGNOSTICS MARKET, BY SAMPLE TYPE, 2025 VS. 2030
- 4.5 MOLECULAR DIAGNOSTICS MARKET, BY TECHNOLOGY, 2025 VS. 2030
- 4.6 MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION, 2025 VS. 2030
- 4.7 MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE, 2025 VS. 2030
- 4.8 MOLECULAR DIAGNOSTICS MARKET, BY CLINICAL APPLICATION, 2025 VS. 2030
- 4.9 MOLECULAR DIAGNOSTICS MARKET, BY END USER, 2025 VS. 2030
- 4.10 MOLECULAR DIAGNOSTICS MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Increasing prevalence of infectious diseases and cancer
- 5.2.1.2 Growing R&D funding
- 5.2.1.3 Surge in technological advancements
- 5.2.1.4 Rising use of point-of-care diagnostic tests
- 5.2.2 RESTRAINTS
- 5.2.2.1 Inadequate reimbursements
- 5.2.2.2 High cost of molecular diagnostic instruments
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Growing significance of companion diagnostics
- 5.2.3.2 Increasing growth opportunities in emerging economies
- 5.2.4 CHALLENGES
- 5.2.4.1 Changing regulatory landscape
- 5.2.4.2 Operational barriers and labor shortage
- 5.2.4.3 Introduction of alternative technologies
- 5.2.1 DRIVERS
- 5.3 PRICING ANALYSIS
- 5.3.1 AVERAGE SELLING PRICE TREND OF MOLECULAR DIAGNOSTIC PRODUCTS
- 5.3.2 AVERAGE SELLING PRICE TREND OF MOLECULAR DIAGNOSTIC REAGENTS & KITS, BY KEY PLAYER
- 5.3.3 AVERAGE SELLING PRICE TREND OF MOLECULAR DIAGNOSTIC PRODUCTS, BY REGION
- 5.4 PATENT ANALYSIS
- 5.4.1 LIST OF MAJOR PATENTS
- 5.5 VALUE CHAIN ANALYSIS
- 5.6 SUPPLY CHAIN ANALYSIS
- 5.7 HS CODES
- 5.7.1 IMPORT SCENARIO (HS CODE 3822)
- 5.7.2 EXPORT SCENARIO (HS CODE 3822)
- 5.8 ECOSYSTEM ANALYSIS
- 5.9 PORTER'S FIVE FORCES ANALYSIS
- 5.9.1 THREAT FROM NEW ENTRANTS
- 5.9.2 THREAT FROM SUBSTITUTES
- 5.9.3 BARGAINING POWER OF BUYERS
- 5.9.4 BARGAINING POWER OF SUPPLIERS
- 5.9.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.10 REGULATORY LANDSCAPE
- 5.10.1 REGULATORY FRAMEWORK
- 5.10.1.1 North America
- 5.10.1.1.1 US
- 5.10.1.1.2 Canada
- 5.10.1.2 Europe
- 5.10.1.2.1 Germany
- 5.10.1.2.2 UK
- 5.10.1.2.3 France
- 5.10.1.2.4 Italy
- 5.10.1.3 Asia Pacific
- 5.10.1.3.1 China
- 5.10.1.3.2 Japan
- 5.10.1.3.3 India
- 5.10.1.4 Latin America
- 5.10.1.4.1 Brazil
- 5.10.1.4.2 Mexico
- 5.10.1.5 Middle East
- 5.10.1.5.1 Africa
- 5.10.1.1 North America
- 5.10.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.10.1 REGULATORY FRAMEWORK
- 5.11 TECHNOLOGY ANALYSIS
- 5.11.1 KEY TECHNOLOGIES
- 5.11.1.1 Polymerase chain reaction
- 5.11.2 COMPLEMENTARY TECHNOLOGIES
- 5.11.2.1 Microarrays
- 5.11.3 ADJACENT TECHNOLOGIES
- 5.11.3.1 Next-generation sequencing
- 5.11.1 KEY TECHNOLOGIES
- 5.12 KEY CONFERENCES & EVENTS, 2025-2026
- 5.13 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 5.14 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.14.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.14.2 BUYING CRITERIA
- 5.15 INVESTMENT AND FUNDING SCENARIO
- 5.16 CASE STUDY ANALYSIS
- 5.16.1 CASE STUDY 1: USING MOLECULAR DIAGNOSTICS TO IDENTIFY ATYPICAL RESPIRATORY INFECTION
- 5.16.2 CASE STUDY 2: IMPLEMENTING RAPID PCR TESTING TO IMPROVE NOROVIRUS DIAGNOSIS AND BED MANAGEMENT
- 5.16.3 CASE STUDY 3: RAPID PCR TESTING STREAMLINED EMERGENCY CARE AND SURGICAL RESOURCE MANAGEMENT IN GERMAN HOSPITAL
- 5.17 IMPACT OF AI/GENERATIVE AI ON MOLECULAR DIAGNOSTICS MARKET
- 5.17.1 INTRODUCTION
- 5.17.2 MARKET POTENTIAL OF AI
- 5.17.2.1 Key use cases
- 5.17.3 IMPLEMENTATION OF AI BY KEY COMPANIES
- 5.17.4 FUTURE OF AI IN MOLECULAR DIAGNOSTICS MARKET
- 5.18 US 2025 TARIFF
- 5.18.1 INTRODUCTION
- 5.18.2 KEY TARIFF RATES
- 5.18.3 PRICE IMPACT ANALYSIS
- 5.18.4 IMPACT ON REGION
- 5.18.4.1 North America
- 5.18.4.2 Europe
- 5.18.4.3 Asia Pacific
- 5.18.5 IMPACT ON END-USE INDUSTRIES
- 5.18.5.1 Hospitals & clinics
- 5.18.5.2 Diagnostic laboratories
6 MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT & SERVICE
- 6.1 INTRODUCTION
- 6.2 REAGENTS & KITS
- 6.2.1 INCREASING AWARENESS ABOUT EARLY DISEASE DETECTION TO PROPEL MARKET
- 6.3 INSTRUMENTS
- 6.3.1 RISING NEED FOR QUICK AND ACCURATE TEST RESULTS TO BOOST MARKET
- 6.4 SERVICES & SOFTWARE
- 6.4.1 GROWING UPTAKE OF ADVANCED INSTRUMENTS TO DRIVE MARKET
7 MOLECULAR DIAGNOSTICS MARKET, BY TEST TYPE
- 7.1 INTRODUCTION
- 7.2 LAB TESTS
- 7.2.1 INCREASING NEED FOR AUTOMATION AND EFFICIENT DISEASE TESTING TO DRIVE MARKET
- 7.3 POC TESTS
- 7.3.1 INCREASED INVESTMENT BY PUBLIC HEALTH AGENCIES AND GOVERNMENTS TO BOOST MARKET
8 MOLECULAR DIAGNOSTICS MARKET, BY SAMPLE TYPE
- 8.1 INTRODUCTION
- 8.2 BLOOD, SERUM, AND PLASMA
- 8.2.1 INCREASING UTILIZATION IN GENETIC TESTING, INFECTIOUS DISEASE DIAGNOSIS, AND CANCER DIAGNOSTICS TO PROPEL MARKET
- 8.3 URINE
- 8.3.1 NEED FOR NON-INVASIVE AND EASY SAMPLE COLLECTION TECHNIQUE TO FUEL MARKET
- 8.4 OTHER SAMPLE TYPES
9 MOLECULAR DIAGNOSTICS MARKET, BY TECHNOLOGY
- 9.1 INTRODUCTION
- 9.2 POLYMERASE CHAIN REACTION
- 9.2.1 RISING USE IN PROTEOMICS AND GENOMICS TO AID GROWTH
- 9.3 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY
- 9.3.1 NEED FOR PROMPT DIAGNOSIS TO STIMULATE GROWTH
- 9.4 DNA SEQUENCING & NEXT-GENERATION SEQUENCING
- 9.4.1 GROWING TECHNOLOGICAL ADVANCEMENTS TO DRIVE MARKET
- 9.5 IN SITU HYBRIDIZATION
- 9.5.1 RISING APPLICATIONS IN DIAGNOSING CHROMOSOMAL ABNORMALITIES AND GENE MAPPING TO BOOST MARKET
- 9.6 DNA MICROARRAYS
- 9.6.1 SURGE IN ADVANCEMENTS IN DESIGN, DENSITY, AND DETECTION TECHNIQUES TO FUEL MARKET
- 9.7 OTHER TECHNOLOGIES
10 MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION
- 10.1 INTRODUCTION
- 10.2 INFECTIOUS DISEASE DIAGNOSTICS
- 10.2.1 SEXUALLY TRANSMITTED DISEASES
- 10.2.1.1 CT/NG
- 10.2.1.1.1 Growing development of novel assays to boost market
- 10.2.1.2 HIV
- 10.2.1.2.1 Increasing blood transfusions and blood donations to propel market
- 10.2.1.3 HPV
- 10.2.1.3.1 Rising cases of cervical cancer to augment growth
- 10.2.1.4 Syphilis
- 10.2.1.4.1 Growing demand for affordable and rapid molecular diagnostic solutions to drive market
- 10.2.1.5 Other sexually transmitted diseases
- 10.2.1.1 CT/NG
- 10.2.2 HEPATITIS
- 10.2.2.1 Hepatitis B
- 10.2.2.1.1 Need for ongoing management and monitoring of virus to drive market
- 10.2.2.2 Hepatitis C
- 10.2.2.2.1 Favorable government initiatives to support growth
- 10.2.2.3 Other hepatitis diseases
- 10.2.2.1 Hepatitis B
- 10.2.3 RESPIRATORY INFECTIOUS DISEASES
- 10.2.3.1 Tuberculosis
- 10.2.3.1.1 Rising focus on reducing tuberculosis deaths to fuel market
- 10.2.3.2 Influenza
- 10.2.3.2.1 Growing strains and subtypes to drive market
- 10.2.3.3 Pharyngitis
- 10.2.3.3.1 Need to differentiate between bacterial and viral infections to fuel growth
- 10.2.3.4 Other respiratory infectious diseases
- 10.2.3.1 Tuberculosis
- 10.2.4 HOSPITAL-ACQUIRED INFECTIONS
- 10.2.4.1 Booming geriatric population to aid growth
- 10.2.5 VECTOR-BORNE DISEASES
- 10.2.5.1 Growing focus on disease surveillance to propel market
- 10.2.6 OTHER INFECTIOUS DISEASES
- 10.2.1 SEXUALLY TRANSMITTED DISEASES
- 10.3 ONCOLOGY TESTING
- 10.3.1 BREAST CANCER
- 10.3.1.1 Increasing need to improve survival rates to support growth
- 10.3.2 COLORECTAL CANCER
- 10.3.2.1 Rising demand for biomarkers to fuel market
- 10.3.3 LUNG CANCER
- 10.3.3.1 Increasing use of predictive biomarkers to drive market
- 10.3.4 PROSTATE CANCER
- 10.3.4.1 Surge in advancements in genomic technologies to aid growth
- 10.3.5 OTHER CANCER TYPES
- 10.3.1 BREAST CANCER
- 10.4 GENETIC TESTING
- 10.4.1 GROWING IMPORTANCE OF DIAGNOSING AND MANAGING RARE GENETIC DISEASES TO PROPEL MARKET
- 10.5 OTHER APPLICATIONS
11 MOLECULAR DIAGNOSTICS MARKET, BY TECHNIQUE
- 11.1 INTRODUCTION
- 11.2 MULTIPLEX TESTING
- 11.2.1 RISING DEMAND FOR QUICK DIAGNOSIS TO PROMOTE GROWTH
- 11.3 SINGLEPLEX TESTING
- 11.3.1 NEED FOR AFFORDABLE TESTING TO AUGMENT GROWTH
12 MOLECULAR DIAGNOSTICS MARKET, BY CLINICAL APPLICATION
- 12.1 INTRODUCTION
- 12.2 DIAGNOSTIC
- 12.2.1 INCREASING CLINICAL NEED FOR CONFIRMATORY TESTING AND TIMELY TREATMENT TO DRIVE MARKET
- 12.3 SCREENING
- 12.3.1 GROWING IMPORTANCE OF EARLY INFECTIOUS DISEASE SCREENING TO PROPEL MARKET EXPANSION
13 MOLECULAR DIAGNOSTICS MARKET, BY END USER
- 13.1 INTRODUCTION
- 13.2 DIAGNOSTIC LABORATORIES
- 13.2.1 INCREASED OUTSOURCING OF LABORATORY DIAGNOSES FOR COST REDUCTION TO AID GROWTH
- 13.3 HOSPITALS & CLINICS
- 13.3.1 GROWING FOCUS ON HEALTHCARE SYSTEMS TO STIMULATE GROWTH
- 13.4 OTHER END USERS
14 MOLECULAR DIAGNOSTICS MARKET, BY REGION
- 14.1 INTRODUCTION
- 14.2 NORTH AMERICA
- 14.2.1 MACROECONOMIC OUTLOOK
- 14.2.2 US
- 14.2.2.1 Presence of advanced healthcare infrastructure and high healthcare expenditures to drive market
- 14.2.3 CANADA
- 14.2.3.1 Growing focus on genomic research to fuel market
- 14.3 EUROPE
- 14.3.1 MACROECONOMIC OUTLOOK
- 14.3.2 GERMANY
- 14.3.2.1 Robust healthcare spending and increasing per capita disposable income to propel market
- 14.3.3 UK
- 14.3.3.1 Expanding community-based diagnostics to drive adoption of molecular diagnostics
- 14.3.4 FRANCE
- 14.3.4.1 Rising R&D expenditure for product launches and development of new technologies to augment growth
- 14.3.5 ITALY
- 14.3.5.1 Growing demographic shift toward elderly population to support market
- 14.3.6 SPAIN
- 14.3.6.1 Surge in demand for genetic and prenatal testing to aid growth
- 14.3.7 RUSSIA
- 14.3.7.1 Increasing access to quality healthcare to boost market growth
- 14.3.8 SWITZERLAND
- 14.3.8.1 Rising healthcare spending for new medications and therapies to drive market
- 14.3.9 REST OF EUROPE
- 14.4 ASIA PACIFIC
- 14.4.1 MACROECONOMIC OUTLOOK
- 14.4.2 CHINA
- 14.4.2.1 Growing public access to modern healthcare to drive market
- 14.4.3 JAPAN
- 14.4.3.1 Universal healthcare reimbursement policy to support market
- 14.4.4 INDIA
- 14.4.4.1 Increasing penetration of medical insurance, hospital setups, and foreign direct investments to fuel market
- 14.4.5 AUSTRALIA
- 14.4.5.1 Rising technological advancements to stimulate growth
- 14.4.6 SOUTH KOREA
- 14.4.6.1 Favorable government initiatives for promoting medical tourism to support growth
- 14.4.7 REST OF ASIA PACIFIC
- 14.5 LATIN AMERICA
- 14.5.1 MACROECONOMIC OUTLOOK
- 14.5.2 BRAZIL
- 14.5.2.1 Universal healthcare access to drive adoption in Brazil
- 14.5.3 MEXICO
- 14.5.3.1 Booming medical tourism industry to aid growth
- 14.5.4 REST OF LATIN AMERICA
- 14.6 MIDDLE EAST & AFRICA
- 14.6.1 MACROECONOMIC OUTLOOK
- 14.6.2 SAUDI ARABIA
- 14.6.2.1 Rising government healthcare expenditure to boost market
- 14.6.3 UAE
- 14.6.3.1 Improvements in healthcare infrastructure to support growth
- 14.6.4 REST OF MIDDLE EAST & AFRICA
15 COMPETITIVE LANDSCAPE
- 15.1 INTRODUCTION
- 15.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 15.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN MOLECULAR DIAGNOSTICS MARKET
- 15.3 REVENUE SHARE ANALYSIS
- 15.4 MARKET SHARE ANALYSIS, 2024
- 15.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 15.5.1 STARS
- 15.5.2 EMERGING LEADERS
- 15.5.3 PERVASIVE PLAYERS
- 15.5.4 PARTICIPANTS
- 15.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 15.5.5.1 Company footprint
- 15.5.5.2 Region footprint
- 15.5.5.3 Product & service footprint
- 15.5.5.4 Sample type footprint
- 15.5.5.5 Application footprint
- 15.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 15.6.1 PROGRESSIVE COMPANIES
- 15.6.2 RESPONSIVE COMPANIES
- 15.6.3 DYNAMIC COMPANIES
- 15.6.4 STARTING BLOCKS
- 15.6.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 15.6.5.1 Detailed list of key startups/SMES
- 15.6.5.2 Competitive benchmarking of startups/SMES
- 15.7 COMPANY VALUATION & FINANCIAL METRICS
- 15.7.1 FINANCIAL METRICS
- 15.7.2 COMPANY VALUATION
- 15.8 BRAND/PRODUCT COMPARISON
- 15.9 COMPETITIVE SCENARIO
- 15.9.1 PRODUCT/SERVICES LAUNCHES AND APPROVALS
- 15.9.2 DEALS
- 15.9.3 EXPANSIONS
16 COMPANY PROFILES
- 16.1 KEY PLAYERS
- 16.1.1 DANAHER CORPORATION
- 16.1.1.1 Business overview
- 16.1.1.2 Products/Services offered
- 16.1.1.3 Recent developments
- 16.1.1.3.1 Product/Service launches and approvals
- 16.1.1.3.2 Deals
- 16.1.1.3.3 Expansions
- 16.1.1.4 MnM view
- 16.1.1.4.1 Right to win
- 16.1.1.4.2 Strategic choices
- 16.1.1.4.3 Weaknesses and competitive threats
- 16.1.2 F. HOFFMANN-LA ROCHE LTD
- 16.1.2.1 Business overview
- 16.1.2.2 Products/Services offered
- 16.1.2.3 Recent developments
- 16.1.2.3.1 Product/Service launches and approvals
- 16.1.2.3.2 Deals
- 16.1.2.4 MnM view
- 16.1.2.4.1 Right to win
- 16.1.2.4.2 Strategic choices
- 16.1.2.4.3 Weaknesses and competitive threats
- 16.1.3 ILLUMINA, INC.
- 16.1.3.1 Business overview
- 16.1.3.2 Products/Services offered
- 16.1.3.3 Recent developments
- 16.1.3.3.1 Product/Service launches and approvals
- 16.1.3.3.2 Deals
- 16.1.3.3.3 Expansions
- 16.1.3.4 MnM view
- 16.1.3.4.1 Right to win
- 16.1.3.4.2 Strategic choices
- 16.1.3.4.3 Weaknesses and competitive threats
- 16.1.4 HOLOGIC, INC.
- 16.1.4.1 Business overview
- 16.1.4.2 Products/Services offered
- 16.1.4.3 Recent developments
- 16.1.4.3.1 Product/Service launches and approvals
- 16.1.4.3.2 Deals
- 16.1.4.4 MnM view
- 16.1.4.4.1 Right to win
- 16.1.4.4.2 Strategic choices
- 16.1.4.4.3 Weaknesses and competitive threats
- 16.1.5 BIOMERIEUX
- 16.1.5.1 Business overview
- 16.1.5.2 Products/Services offered
- 16.1.5.3 Recent developments
- 16.1.5.3.1 Product/Service launches and approvals
- 16.1.5.3.2 Deals
- 16.1.5.4 MnM view
- 16.1.5.4.1 Right to win
- 16.1.5.4.2 Strategic choices
- 16.1.5.4.3 Weaknesses and competitive threats
- 16.1.6 ABBOTT LABORATORIES
- 16.1.6.1 Business overview
- 16.1.6.2 Products/Services offered
- 16.1.6.3 Recent developments
- 16.1.6.3.1 Product/Service launches and approvals
- 16.1.6.3.2 Deals
- 16.1.7 THERMO FISHER SCIENTIFIC INC.
- 16.1.7.1 Business overview
- 16.1.7.2 Products/Services offered
- 16.1.7.3 Recent developments
- 16.1.7.3.1 Product/Service launches and approvals
- 16.1.7.3.2 Deals
- 16.1.8 QIAGEN N.V.
- 16.1.8.1 Business overview
- 16.1.8.2 Products/Services offered
- 16.1.8.3 Recent developments
- 16.1.8.3.1 Product/Service launches and approvals
- 16.1.8.3.2 Deals
- 16.1.8.3.3 Expansions
- 16.1.9 REVVITY, INC.
- 16.1.9.1 Business overview
- 16.1.9.2 Products/Services offered
- 16.1.10 MYRIAD GENETICS, INC.
- 16.1.10.1 Business overview
- 16.1.10.2 Products/Services offered
- 16.1.10.3 Recent developments
- 16.1.10.3.1 Product/Service launches and approvals
- 16.1.10.3.2 Deals
- 16.1.11 SIEMENS HEALTHINEERS AG
- 16.1.11.1 Business overview
- 16.1.11.2 Products/Services offered
- 16.1.11.3 Recent developments
- 16.1.11.3.1 Product/Service launches and approvals
- 16.1.11.3.2 Deals
- 16.1.11.3.3 Expansions
- 16.1.1 DANAHER CORPORATION
- 16.2 OTHER PLAYERS
- 16.2.1 BECTON, DICKINSON AND COMPANY (BD)
- 16.2.2 GRIFOLS, S.A.
- 16.2.3 QUIDELORTHO CORPORATION
- 16.2.4 DIASORIN S.P.A.
- 16.2.5 EXACT SCIENCES CORPORATION
- 16.2.6 GENETIC SIGNATURES
- 16.2.7 AGILENT TECHNOLOGIES, INC.
- 16.2.8 MDXHEALTH
- 16.2.9 BIOCARTIS
- 16.2.10 MEDIGEN BIOTECHNOLOGY CORP.
- 16.2.11 VELA DIAGNOSTICS
- 16.2.12 AMOY DIAGNOSTICS CO., LTD.
- 16.2.13 BRUKER CORPORATION (ELITECHGROUP)
- 16.2.14 MOLBIO DIAGNOSTICS LIMITED
- 16.2.15 GENEOMBIOTECHNOLOGIES
- 16.2.16 SAVYON DIAGNOSTICS
- 16.2.17 UNIOGEN OY
17 APPENDIX
- 17.1 DISCUSSION GUIDE
- 17.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 17.3 CUSTOMIZATION OPTIONS
- 17.4 RELATED REPORTS
- 17.5 AUTHOR DETAILS



















