
|
시장보고서
상품코드
1692467
인실리코 임상시험 시장 : 점유율 분석, 산업 동향 및 통계, 성장 예측(2025-2030년)Global In Silico Clinical Trials - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
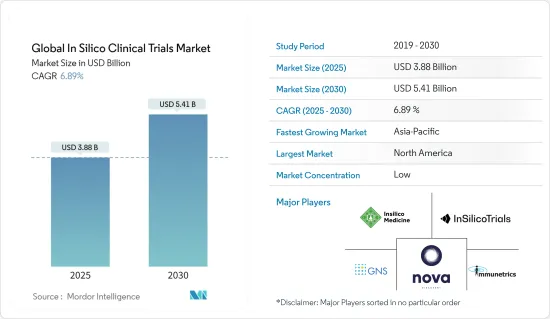
세계의 인실리코 임상시험 시장 규모는 2025년 38억 8,000만 달러로 추정되며, 예측기간 중(2025-2030년) CAGR 6.89%로 확대되어, 2030년에는 54억 1,000만 달러에 달할 것으로 예측되고 있습니다.

COVID-19의 예기치 않은 확산은 인실리코 임상시험 시장에 큰 영향을 미칩니다. 2021년 1월 발행된 뉴스 기사 "Why 2020 Saw The Steady Rise Of In Silico Trials"에서도 COVID-19와 관련된 제약이 임상연구환경에 혼란을 일으키고 있는 가운데, 대기업 제약회사는 기업이 제품개발 노력을 계속할 수 있도록 인실리코 임상시험이나 가상 임상시험에 점점 눈을 돌리게 되고 있다고 설명하고 있습니다. 심각한 계산 모델링과 시뮬레이션 도구를 사용하여 "가상 환자"로 약물을 테스트 할 수 있습니다. 또한 인실리코 시험은 컴퓨터 시뮬레이션을 기반으로 하므로 많은 기존 임상시험을 방해해 온 COVID-19 관련 진행중인 여행이나 사회적 거리 제한에 영향을받지 않습니다.
또한, 약물의 효능과 안전성에 대한 적절한 이해, 수많은 질병의 유병률 증가 및 인실리코 임상시험의 비용 효과가 인실리코 시장을 성장시킵니다. 2021년 Trial지에 게재된 논문 "In silico imaging clinical trials : cheaper, faster, better, safer, and more scalable(인실리코 이미지 임상시험: 보다 싸고, 빠르고, 보다 안전하고, 보다 확장 가능하게)"는 가변성 조정, 샘플 무제한, 환자 위험 없음, 부담 경감 등 인실리코 임상시험이 제공하는 엄청난 이점을 드러내고 있습니다.
게다가, 시장 기업은 M&A나 제품 발표 등의 마케팅 전술에 임하고 있습니다. 2021년 7월, 고형 암에 대한 면역 종양 세포 요법의 선구자인 Kiromic Biopharma, Inc.는 InSilico Solutions를 인수했습니다. Kiromic은 이 인수의 일환으로 바이오인포매틱스와 AI 전문가 팀을 사내에 통합하여 CAR-T 세포 치료와 같은 최첨단 면역 치료제에 최적인 바이오마커를 확인할 수 있는 AI 기술 경쟁에서의 리드를 확대합니다.
인실리코 임상시험의 장점에 대한 이해가 깊어짐에 따라 시장은 해마다 비약적으로 발전하고 있습니다. "VICTRE 테스트"라는 제목으로 미국 식품의약국이 3월 2021일 발표한 웹캐스트에서는 Virtual Imaging Clinical Trial for Regulatory Evaluation(VICTRE 테스트) 시뮬레이션을 위해 2,986명의 인실리코 환자(저선량 X선 시스템 및 컴퓨터 재구성을 사용하여 유방의 3차원 이미지를 생성하는 고급 맘모그래피)의 컴퓨터 시뮬레이션 이미지를 사용한 디지털 맘모그래프 T와 디지털 유방 토모신세시스를 비교한 것으로 설명되어 있습니다.
이와 같은 이유로 토모신세시스 시장은 분석 기간을 통해 성장할 것으로 예상됩니다.
인실리코 임상시험 시장 동향
암 치료 영역은 예측 기간 동안 크게 성장할 것으로 예상된다.
정상적인 암 임상시험은 대부분 비용이 높고 환자에게 해를 끼칠 위험이 큽니다.
2022년 In silico Medicine에 게재된 뉴스에 따르면 필요한 특징을 갖춘 AI 설계 화합물의 실험적 검증을 완료하고 합성 치사성의 표적으로 알려진 유비퀴틴 특이적 프로테아제 1(USP1)을 표적으로 하는 항암 치료제의 전임상 후보(PCC)를 지명했다고 발표했습니다. 합성 치사는 종양 세포의 취약성을 이용해, 정상 세포를 온존한 채로 종양 세포를 사멸시키는 확립된 유전학적 수법이며, 암 치료의 잠재적 분야입니다. In vitro 연구에서는 이 화학물질이 유방암 유전자 변이(BRCA) 종양 세포에서 높은 선택성으로 실질적인 항증식 효과를 나타내는 것으로 밝혀졌습니다.
2021년에 MedRxiv 잡지에 게재된 논문 "In silico cancer immunotherapy trials uncovers the consequences of therapy-specific response patterns for clinical trial design and outcome(인실리코 암 면역치료 임상시험에서 치료별 반응 패턴이 임상시험 설계 및 결과에 미치는 영향을 밝히다)"는 암 면역요법과 인실리코 시험의 이점에 대해 설명하고 있습니다. 면역요법시험의 기초가 되는 생물학적 가정의 타당성을 체크해, 시험 디자인을 지원하는 비용대 효과가 높은 방법입니다. 또, 트레이닝 중의 과학자가 디자인 원리를 이해하는데 도움이 되는 교육 툴로서도 이용할 수 있어 그 결과, 미래의 면역 요법 시험에 있어서 보다 뛰어난 디자인과 더 높은 성공율을 실현할 수 있습니다.
이상과 같은 요인으로부터 암 치료 영역 부문은 예측 기간을 통해 크게 발전할 것으로 예상됩니다.
북미가 인실리코 임상시험 시장을 성장시킬 전망
2021년 인실리코 임상시험 시장에서는 북미가 대부분의 점유율을 차지하고 있었습니다.
주요 제품 출시, 비용 효과, 국내 제조업체의 존재 등이 인실리코 임상시험 시장의 성장에 기여하고 있습니다. GNS Healthcare는 환자별 치료 개입을 시뮬레이트하는 인실리코 환자를 작성하는 인공지능 조직이며, 전립선암 인실리코 환자인 Gemini를 개발 및 발매할 예정 라고 발표했습니다. 이 인실리코 환자는 Tempus사와 공동으로 작성된 것으로, 전체 엑솜 시퀀싱(종양과 정상의 일치), RNAseq, 환자의 치료력, 엔잘타 미드, 아빌라테론, 도세탁셀, 카바지탁셀, 시프레우셀-T, 펨브롤리주맙 등의 현재의 치료법, 관련 치료 라인, 사망률 등의 임상-유전체 데이터를 이용하고 있습니다.
또한 미국 식품의약국이 2022년 1월 발표한 보고서에 따르면 가상 환자 코호트에서 장치를 평가하는 CM&S(Computational Modeling and Simulation)을 채용한 인실리코 임상시험은 미국 식품의약국에 의해 장려되고 있으며, 임상시험을 대체하거나 임상시험을 보강할 것으로 기대되고 있습니다.
인실리코 임상시험 산업 개요
인실리코 임상시험 시장의 경쟁은 중간 정도이며, 여러 선도 기업으로 구성되어 있습니다.
기타 혜택:
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간의 애널리스트 서포트
목차
제1장 서론
- 조사의 전제조건과 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 의약품의 안전성과 효능에 대한 더 나은 이해
- 비용 대비 효과와 시험 중지 가능성의 저감
- 시장 성장 억제요인
- 복잡한 시험 센터를 이용할 수 없는 것과 근사치에 근거한 결과
- Porter's Five Forces 분석
- 신규 참가업체의 위협
- 구매자/소비자의 협상력
- 공급기업의 협상력
- 대체품의 위협
- 경쟁 기업간 경쟁 관계의 강도
제5장 시장 세분화
- 치료영역별
- 암 영역
- 감염증 영역
- 순환기
- 신경학
- 당뇨병
- 기타 치료 영역
- 산업별
- 의료기기
- 의약품
- 페이즈별
- 페이즈 I
- 페이즈 II
- 페이즈 III
- 페이즈 IV
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 프로파일
- Novadiscovery
- Insilico Medicine Inc.
- Dassault Systemes
- GNS Healthcare
- Immunetrics Inc.
- InSilicoTrials Technologies
- Clarivate
- Evotec
- Abzena Ltd
제7장 시장 기회와 앞으로의 동향
JHS 25.05.07The Global In Silico Clinical Trials Market size is estimated at USD 3.88 billion in 2025, and is expected to reach USD 5.41 billion by 2030, at a CAGR of 6.89% during the forecast period (2025-2030).
The unanticipated spread of COVID-19 significantly influences the in silico clinical trials market. Even though the news article "Why 2020 Saw The Steady Rise Of In Silico Trials", published in January 2021, explains that as COVID-19-related constraints continue to cause chaos in the clinical research environment, big pharma companies are increasingly turning to in silico or virtual clinical trials to help companies continue their product development efforts. Before evaluating their drug prospects in humans, companies can use advanced computational modeling and simulation tools to test them in "virtual patients." Additionally, since the in silico studies are based on computer simulations, they are unaffected by the ongoing COVID-19-related travel and social distancing restrictions that have hampered many traditional trials.
Furthermore, the proper understanding of drug efficacy and safety, the growing prevalence of numerous diseases, and the cost-effectiveness of in silico clinical trials make the in silico market grow. The article "In silico imaging clinical trials: cheaper, faster, better, safer, and more scalable," published in the Trial journal in 2021, reveals the tremendous advantages offered by in silico clinical trials, such as adjustable variability, unlimited samples, no patient risk, and less burden. These advantages, in turn, lead to high demand for in silico clinical trial market.
Furthermore, market players are engaged in marketing tactics such as mergers and acquisitions and product launches. For instance, in July 2021, Kiromic Biopharma, Inc., a pioneer in Immuno oncology cellular therapy for solid tumors, acquired InSilico Solutions. Kiromic will integrate a team of bioinformatics and AI professionals in-house as part of this deal, extending its lead in the race for AI technology that can identify the best biomarkers for cutting-edge immunotherapeutics like CAR-T cell treatment.
Increased understanding of the benefits of in silico clinical trials has led the market to develop exponentially over the ages. The webcast published by the United States Food and Drug Administration under the title "The VICTRE trial: an in-silico replica of a clinical trial for evaluating digital breast tomosynthesis as a replacement for full-field digital mammography" on March 2021 explains the simulated Virtual Imaging Clinical Trial for Regulatory Evaluation (VICTRE trial) compared digital mammography and digital breast tomosynthesis using computer-simulated imaging of 2,986 in silico patients (an advanced type of mammography that generates three-dimensional images of the breasts using a low-dose x-ray system and computer reconstructions). All breast sizes and lesion types showed an improved lesion detection performance favoring tomosynthesis. The improved tomosynthesis performance was in line with findings from a comparison trial including real patients and radiologists.
As a result of the reasons outlined, the explored market is anticipated to grow throughout the analysis period. However, since human subjects are not involved, the results are based on an approximation that can restrain market growth in silico clinical trials.
In Silico Clinical Trials Market Trends
Oncology Therapeutic Area is Expected to Witness a Significant Growth Over the Forecast Period
Regular cancer clinical trials are mostly at a high cost and have a significant risk of causing harm to patients. The demand for cancer in silico clinical trials is mostly driven by these considerations. Furthermore, technological advancements such as using artificial intelligence (AI) in cancer computer simulation studies to improve medication knowledge, safety, and efficacy are boosting the market's growth.
As per the news published in In silico Medicine in 2022, they announced they had completed experimental validation of AI-designed compounds with the requisite features and have nominated a preclinical candidate (PCC) for anti-cancer therapeutics targeting ubiquitin-specific protease 1 (USP1), a known synthetic lethality target. Synthetic lethality, a well-established genetic technique to exploit vulnerabilities in tumor cells to trigger tumor cell death while sparing normal cells, is a potential field of cancer therapy. When used against a wide spectrum of tumor lineages, Insilico's preclinical candidate molecule showed promising results. In vitro studies revealed that the chemical has a substantial antiproliferative effect in breast cancer gene mutant (BRCA) tumor cells with high selectivity.
The article published in MedRxiv journal under the heading "In silico cancer immunotherapy trials uncovers the consequences of therapy-specific response patterns for clinical trial design and outcome" in 2021 explains the advantages of cancer immunotherapy coupled with in silico trials. In the study, they point out that, for immuno-oncology, in silico studies have major implications. They offer a cost-effective way to check the validity of the biological assumptions that underpin immunotherapy trials and aid in their design. It can also be used as a teaching tool that can help scientists in training understand design principles, resulting in better designs and higher success rates in future immunotherapy studies.
As a result of the stated factors, the oncology therapeutic area segment is expected to develop significantly throughout the forecast period.
North America is Expected to Grow the In Silico Clinical Trials Market
North America had the majority share in the in silico clinical trials market in 2021. The emergence of this region can be attributed to an increase in understanding of the benefits of in silico trials and reduced side effects that have been raised from human trials.
Key product releases, cost-effectiveness, and the presence of manufacturers in the country are all factors that have contributed to the growth of the in silico clinical trials market. According to the press release on May 2021, Massachusetts-based GNS Healthcare, an artificial intelligence organization that produces in silico patients that simulate therapeutic intervention on a patient-by-patient basis, announced that they are planning to develop and launch Gemini, which is an in silico Patient for Prostate Cancer. This in silico patient was created in collaboration with Tempus and is powered by clinico-genomic data, including whole-exome sequencing (tumor/normal match), RNAseq, patient treatment history, current treatments like Enzalutamide, Abiraterone, Docetaxel, Cabazitaxel, Sipuleucel-T, and Pembrolizumab, as well as related lines of treatment and mortality.
Moreover, according to the report published by the United States Food and Drug Administration on January 2022, describe that In silico clinical trials employing Computational Modeling and Simulation (CM&S), in which a device is evaluated on a cohort of virtual patients, are encouraged by the United States Food and Drug Administration and are expected to replace or augment clinical studies. However, the use of Computational Modeling and Simulation to support regulatory filings is hampered by a lack of or contradictory information on their reliability. The analyzed market is expected to grow in the North American region due to the factors mentioned above.
In Silico Clinical Trials Industry Overview
The in silico clinical trial market is moderately competitive and consists of several major players. The competitive landscape includes an analysis of a few international and local companies that hold market shares and are well known. Include Novadiscovery, Insilico Medicine, Inc., GNS Healthcare, InSilicoTrials Technologies, and Immunetrics Inc., among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Better Understanding of the Safety and Efficacy of a Drug
- 4.2.2 Cost-effectiveness and Less Chances of Termination of the Trial
- 4.3 Market Restraints
- 4.3.1 Unavailability of Complex Testing Centers and Results Based on Approximation
- 4.4 Porter's Five Force Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Therapeutic Area
- 5.1.1 Oncology
- 5.1.2 Infectious Disease
- 5.1.3 Cardiology
- 5.1.4 Neurology
- 5.1.5 Diabetes
- 5.1.6 Other Therapeutic Areas
- 5.2 By Industry
- 5.2.1 Medical Devices
- 5.2.2 Pharmaceutical
- 5.3 By Phase
- 5.3.1 Phase I
- 5.3.2 Phase II
- 5.3.3 Phase III
- 5.3.4 Phase IV
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle-East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle-East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Novadiscovery
- 6.1.2 Insilico Medicine Inc.
- 6.1.3 Dassault Systemes
- 6.1.4 GNS Healthcare
- 6.1.5 Immunetrics Inc.
- 6.1.6 InSilicoTrials Technologies
- 6.1.7 Clarivate
- 6.1.8 Evotec
- 6.1.9 Abzena Ltd











