
|
시장보고서
상품코드
1762523
분자진단 시장 : 업계 동향과 세계 예측 - 검사 유형별, 오퍼링 유형별, 샘플 유형별, 기술 유형별, 치료 영역별, 최종사용자별, 지역별Molecular Diagnostics Market: Industry Trends and Global Forecasts - Distribution by Test Type, Type of Offering, Type of Sample, Type of Technology, Therapeutic Area, End Users, and Geographical Regions |
||||||
분자진단 시장 : 개요
세계의 분자진단 시장 규모는 2035년까지의 예측 기간 중 6.2%의 CAGR로 확대하며, 현재 159억 달러에서 2035년까지 309억 달러로 성장할 것으로 예측됩니다.
시장 세분화에서는 시장 규모와 기회 분석을 다음과 같은 매개 변수로 구분합니다.
검사 유형
- 실험실 검사
- POC(Point-Of-Care) 검사
제공 제품 유형
- 시약
- 검사 장비
- 서비스
샘플 유형
- 혈액, 혈청, 혈장, 혈장
- 소변
- 기타
기술 유형
- 중합효소 연쇄반응(PCR)
- in situ 하이브리드화
- 등온핵산증폭기술
- 차세대 시퀀싱
- 마이크로어레이
- 질량분석
- 기타
치료 영역
- 심혈관 질환
- 유전자 질환
- 감염성 질환
- 신경질환
- 종양 질환
- 기타
최종사용자
- 병원
- 연구소
- 기타
주요 지역
- 북미(미국, 캐나다)
- 유럽(오스트리아, 벨기에, 프랑스, 독일, 이탈리아, 네덜란드, 폴란드, 스페인, 스위스, 영국, 기타)
- 아시아(중국, 인도, 인도네시아, 일본, 싱가포르, 한국, 태국, 기타)
- 라틴아메리카(아르헨티나, 브라질, 멕시코, 멕시코, 기타)
- 중동/북아프리카(이집트, 이스라엘, 사우디아라비아, 기타)
- 기타 지역(호주, 뉴질랜드)
분자진단 시장 : 성장과 동향
분자진단 검사는 유전체과 프로테옴의 생물학적 마커를 분석하는 데 사용되는 첨단 기술 및 툴입니다. 이러한 진단 솔루션은 질병을 감지하고 모니터링하며, 유전자 이상을 식별하고, 개인별 맞춤 치료 계획을 세우는 데 필수적입니다. 분자진단 분야에서 사용되는 주요 기술로는 중합효소연쇄반응, 차세대 염기서열분석, 마이크로어레이 등이 있으며, PCR은 미량의 DNA와 RNA를 증폭하고 검출할 수 있는 특이성이 높은 기술인 반면, NGS는 전체 유전체의 고처리량 염기서열분석을 가능케 합니다. 이처럼 분자진단 솔루션은 암질환, 감염질환, 유전자 검사, 맞춤의료 등 다양한 의료 분야에서 매우 중요한 역할을 하고 있습니다. 이러한 솔루션은 진단의 정확성을 높이고 맞춤형 치료 전략을 가능하게 하고 지원함으로써 궁극적으로 진단 결과를 개선하고 공중 보건을 향상시키는 것을 목표로 하고 있습니다. 또한 의료 의사결정의 70% 이상이 임상 검사 결과를 기반으로 이루어지고 있다는 사실은 환자 치료에서 이러한 진단 툴이 얼마나 중요한지를 반영합니다.
또한 분자진단 솔루션이 제공하는 빠른 검사, 시간 단축, 신속한 의사결정 등 여러 가지 장점으로 인해 시장은 예측 기간 중 건전한 CAGR로 성장할 것으로 예측됩니다.
분자진단 시장 : 주요 인사이트
이 보고서는 분자진단 시장의 현황을 조사하고 잠재적인 성장 기회를 파악합니다. 이 보고서의 주요 조사 결과는 다음과 같습니다.
- 분자진단 분야는 다양한 진단 용도를 제공하기 위해 다양한 유형의 첨단 기술을 활용하는 진입업체들의 역동적인 시장 상황이 특징입니다.
- 본 분석의 대상인 대부분의 대기업은 1951-2000년에 설립되었으며, 그 중 60%가 북미에 기반을 두고 있습니다.
- 로슈는 분자진단 솔루션의 다양한 포트폴리오와 최근 수년간의 호실적을 바탕으로 이 분야의 주요 기업 중 가장 강력한 기업으로 부상했습니다.
- 분자진단 시장의 다양한 동향의 영향을 조사하기 위해 당사는 독자적인 조사 방법을 개발하여 Porter's Five Forces의 틀에 따라 다양한 매개 변수를 분석했습니다.
- 분자진단 시장은 예방적 헬스케어에 대한 인식이 높아짐에 따라 성장하고 있지만, 복잡한 규제 대응 등 업계 진출기업에게는 여전히 큰 걸림돌로 작용하고 있습니다.
- 전 세계에서 만성질환의 유병률 증가에 힘입어 분자진단 시장은 예측 기간 중 6.2%의 견고한 성장률을 나타낼 것으로 예측됩니다.
- 예상되는 미래 기회는 검사 유형, 샘플 유형, 치료 영역, 최종사용자 등 여러 부문에 잘 분산될 것으로 예측됩니다.
분자진단 시장 : 주요 부문
검사 유형에 따라 시장은 실험실 검사와 현장 검사로 구분됩니다. 현재 분자진단 시장에서 실험실 검사 부문이 가장 큰 점유율을 차지하고 있습니다. 이러한 추세는 앞으로도 변하지 않을 것으로 예측됩니다. 또한 현장 검사 분야의 분자진단 시장은 예측 기간 중 가장 높은 시장 성장 잠재력을 보일 것으로 예측됩니다.
제품 유형별로는 시약이 세계 분자진단 시장에서 가장 빠르게 성장하고 있습니다.
시약, 기기, 서비스로 구분됩니다. 현재 시약 부문은 세계 분자진단 시장에서 가장 큰 점유율을 차지하고 있습니다. 또한 시약은 빈번한 보충이 필요하며, 이는 경상 매출에 기여하므로 시약 부문은 예측 기간 중 더 높은 CAGR로 성장할 것으로 예측됩니다.
샘플 유형에 따라 시장은 혈액, 혈청 및 혈장, 소변, 기타 검체로 구분됩니다. 현재 혈액, 혈청 및 혈장 부문이 세계 분자진단 시장에서 가장 큰 비중을 차지하고 있습니다. 그러나 소변 부문은 예측 기간 중 더 높은 CAGR로 성장할 것으로 예측됩니다.
기술 유형별로 시장은 중합효소연쇄반응(PCR), in situ hybridization, 등온핵산증폭기술, 차세대 시퀀싱, 마이크로어레이, 질량분석, 기타로 구분됩니다. 중합효소연쇄반응(PCR) 분야가 전체 시장의 주요 촉진요인이 될 것으로 예상되지만, 차세대 시퀀싱 분야의 세계 분자진단 시장이 상대적으로 높은 CAGR로 성장할 가능성이 높다는 점은 주목할 만합니다. 이는 높은 처리량, 정확도 향상, 여러 유전자의 동시 염기서열 분석 기능 등 차세대 시퀀서가 제공하는 몇 가지 장점에 기인하는 것으로 보입니다.
시장은 치료 영역별로 심혈관질환, 유전질환, 감염질환, 신경질환, 종양질환, 기타로 구분됩니다. 현재 감염질환 분야가 분자진단 의약품 시장에서 가장 큰 점유율을 차지하고 있습니다. 또한 신경질환 부문은 예측 기간 중 가장 높은 성장 잠재력을 보이며 다른 부문에 비해 높은 CAGR로 성장할 것으로 예측됩니다.
최종사용자별로 세계 시장은 병원, 연구소, 기타로 구분됩니다. 현재 병원 부문이 가장 큰 시장 점유율을 차지하고 있습니다. 그러나 실험실용 분자진단 시장은 향후 수년간 큰 폭의 성장이 예상됩니다.
주요 지역별로 시장은 북미, 유럽, 아시아, 아시아, 라틴아메리카, 중동 및 아프리카, 기타 라틴아메리카로 구분됩니다. 현재 북미가 세계 분자진단 의약품 시장을 독점하고 있으며, 가장 큰 매출 점유율을 차지하고 있습니다. 또한 아시아태평양 시장은 향후 더 높은 CAGR로 성장할 것으로 예측됩니다.
분자진단 시장의 참여 기업 예
- Abbott Laboratories
- Agilent Technologies
- Becton Dickinson
- BGI Genomics
- bioMerieux
- Bio-Rad
- Danaher
- DiaSorin
- Grifols
- Hologic
- Illumina
- Qiagen
- QuidelOrtho
- Revvity
- Roche
- Sansure
- Seegene
- Siemens Healthineers
- Sysmex
- Thermo Fisher Scientific
목차
제1장 배경
제2장 조사 방법
제3장 경제적 및 기타 프로젝트 특유 고려 사항
제4장 개요
제5장 서론
- 분자진단의 개요
- 분자진단 솔루션에 채택되고 있는 주요 기술
- 분자진단 분야의 과제
- 분자진단 분야의 최근 동향
- 분자진단 분야의 전망
제6장 시장 영향 분석 : 촉진요인, 억제요인, 기회, 과제
제7장 세계의 분자진단 시장
- 주요 전제와 조사 방법
- 세계의 분자진단 시장(-2035년)
제8장 분자진단 시장(검사 유형별)
- 시장 변수 분석
- 분자진단 시장 : 검사 유형별
- 2035년까지의 임상 검사용 분자진단 시장
- 2035년까지의 POC 검사용 분자진단 시장
제9장 분자진단 시장(오퍼링 유형별)
- 시장 변수 분석
- 분자진단 시장 : 오퍼링 유형별
제10장 분자진단 시장(샘플 유형별)
- 시장 변수 분석
- 분자진단 시장 : 샘플 유형별
제11장 분자진단 시장(기술별)
- 시장 변수 분석
- 분자진단 시장 : 기술 유형별
제12장 분자진단 시장(치료 영역별)
- 시장 변수 분석
- 분자진단 시장 : 치료 영역별
제13장 분자진단 시장(최종사용자별)
- 시장 변수 분석
- 분자진단 시장 : 최종사용자별
제14장 분자진단 시장(지역별)
- 시장 변수 분석
- 분자진단 시장 : 지역별
제15장 분자진단 시장(주요 기업별)
- 분자진단 시장 : 연간 판매량별 주요 기업
제16장 시장 개요 : 주요 분자진단 솔루션 프로바이더
- 분자진단 솔루션 : 시장 구도
- 분자진단 : 솔루션 프로바이더의 상황
제17장 기업 경쟁력 분석 : 분자진단 솔루션 프로바이더
- 평가 조사 방법과 주요 파라미터
- 분자진단 솔루션 프로바이더 : 대기업의 경쟁력 분석
- 분자진단 솔루션 프로바이더 : 대기업의 경쟁력 분석
- 벤치마크 분석 : 주요 분자진단 솔루션 프로바이더
제18장 기업 개요 : 북미에 본사를 둔 분자진단 솔루션 프로바이더
- 상세한 기업 개요
- Abbott
- Agilent Technologies
- BD
- Danaher
- Thermo Fisher Scientific
- 기타 기업 개요
- Bio-Rad
- Illumina
- Hologic
- PerkinElmer
- QuidelOrtho
제19장 기업 개요 : 유럽에 본사를 둔 분자진단 솔루션 프로바이더
- 상세한 기업 개요
- bioMerieux
- Grifols
- Roche
- Siemens Healthineers
- 기타 기업 개요
- DiaSorin
- Qiagen
제20장 기업 개요 : 아시아에 본사를 둔 분자진단 솔루션 프로바이더
- 상세한 기업 개요
- Sysmex
- 기타 기업 개요
- BGI Genomics
- Sansure
- Seegene
제21장 Porter's Five Forces 분석
제22장 부록 I : 표형식 데이터
제23장 부록 II : 기업 및 조직 리스트
KSA 25.07.10MOLECULAR DIAGNOSTICS MARKET: OVERVIEW
As per Roots Analysis, the global molecular diagnostics market is estimated to grow from USD 15.9 billion in the current year to USD 30.9 billion by 2035, at a CAGR of 6.2% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Test Type
- Laboratory Testing
- Point-of-Care Testing
Type of Offering
- Reagents
- Instruments
- Services
Type of Sample
- Blood, Serum and Plasma
- Urine
- Others
Type of Technology
- Polymerase Chain Reaction (PCR)
- In situ Hybridization
- Isothermal Nucleic Acid Amplification Technology
- Next Generation Sequencing
- Microarrays
- Mass Spectrometry
- Others
Therapeutic Area
- Cardiovascular Diseases
- Genetic Diseases
- Infectious Diseases
- Neurological Diseases
- Oncological Diseases
- Others
End Users
- Hospitals
- Laboratories
- Others
Key Geographical Regions
- North America (US, Canada)
- Europe (Austria, Belgium, France, Germany, Italy, Netherlands, Poland, Spain, Switzerland, UK, Rest of the Europe)
- Asia (China, India, Indonesia, Japan, Singapore, South Korea, Thailand, Rest of Asia)
- Latin America (Argentina, Brazil, Mexico, Rest of Latin America)
- Middle East and North Africa (Egypt, Israel, Saudi Arabia, Rest of Middle East and North Africa)
- Rest of the World (Australia and New Zealand)
MOLECULAR DIAGNOSTICS MARKET: GROWTH AND TRENDS
Molecular diagnostic tests are advanced techniques and tools used to analyze biological markers in the genome and proteome. These diagnostic solutions are essential for detecting and monitoring diseases, identifying genetic abnormalities, and guiding personalized treatment plans. The primary technologies used in the molecular diagnostics domain include polymerase chain reaction, next-generation sequencing and microarrays. While PCR is a highly specific technique that enables the amplification and detection of trace amounts of DNA or RNA, NGS allows for high-throughput sequencing of entire genomes. Thus, molecular diagnostic solutions are pivotal across various medical fields, including oncological disorders, infectious diseases, genetic testing, and personalized medicine. These solutions enhance the accuracy of diagnosis, enable and support tailored treatment strategies aiming to ultimately improve diagnostic outcomes and advancing public health. In addition, it is worth mentioning that more than 70% of the healthcare decisions are made based on laboratory test results, which reflects the importance of such diagnostic tools in patient care.
Further, owing to the several benefits offered by these molecular diagnostic solutions, such as providing rapid testing, reducing turnaround times and enabling quicker decision-making, the market is expected to grow at a healthy compounded annual growth rate (CAGR) during the forecast period.
MOLECULAR DIAGNOSTICS MARKET: KEY INSIGHTS
The report delves into the current state of the molecular diagnostics market and identifies potential growth opportunities within industry. Some key findings from the report include:
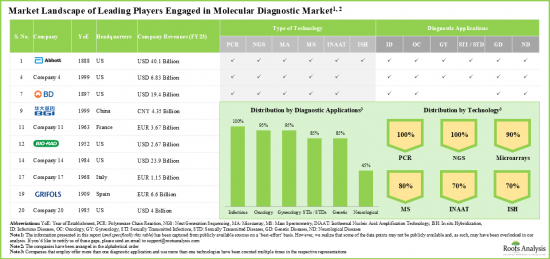
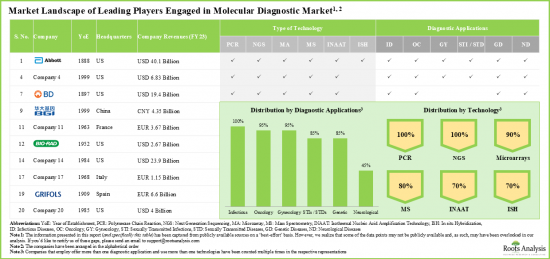
- The molecular diagnostic domain features a dynamic market landscape of players that utilize various types of advanced technologies in order to offer a variety of diagnostic applications.
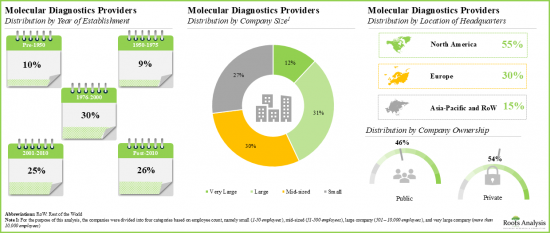
- A number of leading players considered in this analysis were established during 1951 to 2000; 60% of such players are based in North America.
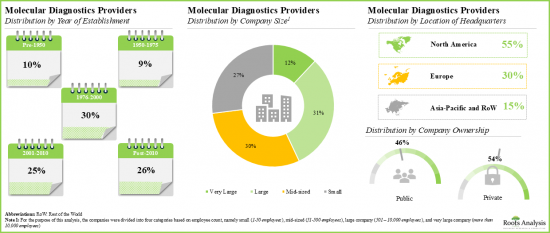
- Owing to its diverse portfolio of molecular diagnostics solutions and strong financial performance in recent fiscal year, Roche emerged as the most competent company among the leading players in this domain.
- In order to study the impact of various trends in the molecular diagnostics market, we developed our proprietary research methodology to analyze different parameters under Porter's Five Forces framework.
- The molecular diagnostics market is fueled by growing awareness towards preventive healthcare; however, factors, such as navigating through regulatory complexities remain significant hurdles for industry players.
- Driven by the increasing prevalence of chronic disorders across the globe, the global molecular diagnostics market is expected to grow at a healthy growth rate of 6.2% during the forecast period.
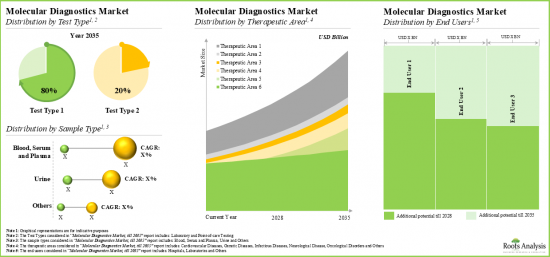
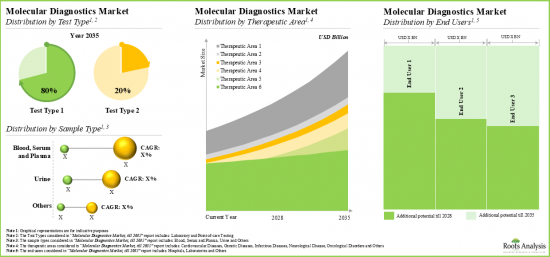
- The anticipated future opportunity is expected to be well distributed across multiple segments, such as test type, sample type, therapeutic area, and end users.
MOLECULAR DIAGNOSTICS MARKET: KEY SEGMENTS
Laboratory Testing Segment holds the Largest Share of the Molecular Diagnostics Market
Based on the test type, the market is segmented into laboratory testing and point-of-care testing. At present, the laboratory testing segment holds the maximum share of the molecular diagnostics market. This trend is likely to remain the same in the coming future. Further, the molecular diagnostics market for point-of-care testing segment is expected to show the highest market growth potential during the forecast period.
By Type of Offering, Reagents is the Fastest Growing Segment of the Global Molecular Diagnostics Market
Based on the type of offering, the market is segmented into reagents, instruments and services. At present, the reagents segment holds the maximum share of the global molecular diagnostics market. Further, owing to the fact that reagents are required to be replenished frequently, which contributes to the recurrent revenues, the market for reagents segment is expected to grow at a higher CAGR during the forecast period.
By Type of Sample, Blood, Serum and Plasma Segment Accounts for the Largest Share of the Global Molecular Diagnostics Market
Based on the type of sample, the market is segmented into blood, serum and plasma, urine, and other samples. Currently, the blood, serum and plasma segment capture the highest proportion of the global molecular diagnostics market. However, the urine segment is expected to grow at a higher CAGR during the forecast period.
The Polymerase Chain Reaction (PCR) Segment by Type of Technology Occupy the Largest Share of the Molecular Diagnostics Market
Based on the type of technology, the market is segmented into Polymerase Chain Reaction (PCR), in situ hybridization, isothermal nucleic acid amplification technology, next generation sequencing, microarrays, mass spectrometry and others. While the polymerase chain reaction (PCR) segment is expected to be the primary driver of the overall market, it is worth highlighting that the global molecular diagnostics market for next generation sequencing segment is likely to grow at a relatively higher CAGR. This can be attributed to the several benefits offered by next generation sequencing, such as high-throughput, improved accuracy, and the capability to simultaneously sequence multiple genes.
By Therapeutic Area, Infectious Disease Segment is Likely to Dominate the Molecular Diagnostics Market
Based on the therapeutic area, the market is segmented into cardiovascular diseases, genetic diseases, infectious diseases, neurological diseases, oncological diseases and others. At present the infectious diseases segment holds the maximum share of the molecular diagnostics market. Additionally, the neurological diseases segment is expected to show the highest growth potential during the forecast period, growing at a higher CAGR, compared to the other segments.
Currently, Hospitals Segment Holds the Largest Share of the Molecular Diagnostics Market
Based on end users, the global market is segmented into hospitals, laboratories, and others. Currently, the hospitals segment holds the largest market share. However, the molecular diagnostics market for laboratories segment is expected to witness substantial growth in the coming years.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia, Latin America, Middle East and North Africa, and Rest of the World. Currently, North America dominates the global molecular diagnostics market and accounts for the largest revenue share. Further, the market in Asia-Pacific is likely to grow at a higher CAGR in the coming future.
Example Players in the Molecular Diagnostics Market
- Abbott Laboratories
- Agilent Technologies
- Becton Dickinson
- BGI Genomics
- bioMerieux
- Bio-Rad
- Danaher
- DiaSorin
- Grifols
- Hologic
- Illumina
- Qiagen
- QuidelOrtho
- Revvity
- Roche
- Sansure
- Seegene
- Siemens Healthineers
- Sysmex
- Thermo Fisher Scientific
MOLECULAR DIAGNOSTICS MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global molecular diagnostics market, focusing on key market segments, including [A] test type, [B] type of offering, [C] type of sample, [D] type of technology, [E] therapeutic area, [F] end users and [D] key geographical regions.
- Market Impact Analysis: A thorough analysis of various factors, such as drivers, restraints, opportunities, and existing challenges that are likely to impact market growth.
- Market Landscape: A comprehensive evaluation of the leading molecular diagnostics companies, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] company ownership and [D] location of the headquarters. Further, the section includes a comprehensive evaluation of molecular diagnostic solutions, focusing on the parameters, such as [A] type of technology used and [B] diagnostic applications.
- Company Competitiveness Analysis: A comprehensive competitive analysis of molecular diagnostic companies, examining factors, such as [A] years of experience and [B] company competitiveness.
- Regulatory Landscape for Medical Devices: A comprehensive discussion of the various guidelines established by major regulatory bodies for medical device approval across different countries. Additionally, a multi-dimensional bubble analysis was done, focusing on the comparison of contemporary regulatory scenario in key geographies across the globe.
- Company Profiles: In-depth profiles of key players that specialize in molecular diagnostic solutions, focusing on [A] overview of the company, [B] financial information, [C] molecular diagnostic offerings and [D] recent developments and an informed future outlook.
- Porter's Five Forces Analysis: A qualitative assessment of Porter's Five Forces framework based on the five competitive forces, including [A] threats to new entrants, [B] bargaining power of product providers, [C] bargaining power of buyers, [D] threat of substitute products and [E] rivalry among existing competitors.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. BACKGROUND
- 1.1. Context
- 1.2. Project Objectives
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.3. Project Methodology
- 2.4. Forecast Methodology
- 2.5. Robust Quality Control
- 2.6. Key Market Segmentations
- 2.7. Key Factors
- 2.7.1. Demographics
- 2.7.2. Economic Factors
- 2.7.3. Government Regulations
- 2.7.4. Supply Chain
- 2.7.5. COVID Impact
- 2.7.6. Market Access
- 2.7.7. Healthcare Policies
- 2.7.8. Industry Consolidation
3. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Future
- 3.2.2. Currency Coverage and Foreign Exchange Rate
- 3.2.2.1. Major Currencies Affecting the Market
- 3.2.2.2. Factors Affecting Currency Fluctuations and Foreign Exchange Rates
- 3.2.2.3. Impact of Foreign Exchange Rate Volatility on the Market
- 3.2.2.4. Strategies for Mitigating Foreign Exchange Risk
- 3.2.3. Trade Policies
- 3.2.3.1. Impact of Trade Barriers on the Market
- 3.2.3.2. Strategies for Mitigating the Risks Associated with Trade Barriers
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. EXECUTIVE SUMMARY
5. INTRODUCTION
- 5.1. Overview of Molecular Diagnostics
- 5.2. Key Technologies Employed in Molecular Diagnostic Solution
- 5.3. Challenges in the Molecular Diagnostics Domain
- 5.4. Recent Developments in the Molecular Diagnostics Domain
- 5.5. Future Perspective in the Molecular Diagnostics Domain
6. MARKET IMPACT ANALYSIS: DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES
- 6.1. Market Drivers
- 6.2. Market Restraints
- 6.3. Market Opportunities
- 6.4. Market Challenges
7. GLOBAL MOLECULAR DIAGNOSTICS MARKET
- 7.1. Key Assumptions and Methodology
- 7.2. Global Molecular Diagnostics Market, Till 2035
- 7.2.1. Scenario Analysis
- 7.2.1.1. Conservative Scenario
- 7.2.1.2. Optimistic Scenario
- 7.2.1. Scenario Analysis
8. MOLECULAR DIAGNOSTICS MARKET, BY TEST TYPE
- 8.1. Market Movement Analysis
- 8.2. Molecular Diagnostics Market: Distribution by Test Type
- 8.2.1. Molecular Diagnostics Market for Laboratory Testing, Till 2035
- 8.2.2. Molecular Diagnostics Market for Point-of-Care Testing, Till 2035
9. MOLECULAR DIAGNOSTICS MARKET, BY TYPE OF OFFERING
- 9.1. Market Movement Analysis
- 9.2. Molecular Diagnostics Market: Distribution by Type of Offering
- 9.2.1. Molecular Diagnostics Market for Instruments, Till 2035
- 9.2.1.1. Molecular Diagnostics Market for In-house Instruments, Till 2035
- 9.2.1.2. Molecular Diagnostics Market for Outsourced Instruments, Till 2035
- 9.2.2. Molecular Diagnostics Marlet for Reagents, till 2035
- 9.2.3. Molecular Diagnostics Market for Services, till 2035
- 9.2.1. Molecular Diagnostics Market for Instruments, Till 2035
10. MOLECULAR DIAGNOSTICS MARKET, BY SAMPLE TYPE
- 10.1. Market Movement Analysis
- 10.2. Molecular Diagnostics Market: Distribution by Sample Type
- 10.2.1. Molecular Diagnostics Market for Blood, Serum and Plasma, till 2035
- 10.2.2. Molecular Diagnostics Market for Urine, till 2035
- 10.2.3. Molecular Diagnostics Market for Other Samples, till 2035
11. MOLECULAR DIAGNOSTICS MARKET, BY TYPE OF TECHNOLOGY
- 11.1. Market Movement Analysis
- 11.2. Molecular Diagnostics Market: Distribution by Type of Technology
- 11.2.1. Molecular Diagnostics Market for PCR, till 2035
- 11.2.2. Molecular Diagnostics Market for In Situ Hybridization, till 2035
- 11.2.3. Molecular Diagnostics Market for Isothermal Nucleic Acid Amplification Technology, till 2035
- 11.2.4. Molecular Diagnostics Market for Next Generation Sequencing, till 2035
- 11.2.5. Molecular Diagnostics Market for Microarrays, till 2035
- 11.2.6. Molecular Diagnostics Market for Mass Spectrometry, till 2035
- 11.2.7. Molecular Diagnostics Market for Other Technologies, till 2035
12. MOLECULAR DIAGNOSTICS MARKET, BY THERAPEUTIC AREA
- 12.1. Market Movement Analysis
- 12.2. Molecular Diagnostics Market: Distribution by Therapeutic Area
- 12.2.1. Molecular Diagnostics Market for Infectious Diseases, till 2035
- 12.2.1.1. Molecular Diagnostics Market for COVID-19, till 2035
- 12.2.1.2. Molecular Diagnostics Market for Respiratory Infections (Excluding COVID-19), till 2035
- 12.2.1.3. Molecular Diagnostics Market for Healthcare-associated Infections, till 2035
- 12.2.1.4. Molecular Diagnostics Market for Hepatitis, till 2035
- 12.2.1.5. Molecular Diagnostics Market for HIV, till 2035
- 12.2.1.6. Molecular Diagnostics Market for Sexually Transmitted Diseases, till 2035
- 12.2.1.7. Molecular Diagnostics Market for Other Infectious Diseases, till 2035
- 12.2.2. Molecular Diagnostics Market for Oncological Disorders, till 2035
- 12.2.2.1. Molecular Diagnostics Market for Lung Cancer, till 2035
- 12.2.2.2. Molecular Diagnostics Market for Breast Cancer, till 2035
- 12.2.2.3. Molecular Diagnostics Market for Colorectal Cancer, till 2035
- 12.2.2.4. Molecular Diagnostics Market for Prostate Cancer, till 2035
- 12.2.2.5. Molecular Diagnostics Market for Gastric Cancer, till 2035
- 12.2.2.6. Molecular Diagnostics Market for Other Oncological Disorders, till 2035
- 12.2.3. Molecular Diagnostics Market for Cardiovascular Diseases, till 2035
- 12.2.4. Molecular Diagnostics Market for Neurological Diseases, till 2035
- 12.2.5. Molecular Diagnostics Market for Genetic Diseases, till 2035
- 12.2.6. Molecular Diagnostics Market for Other Therapeutic Areas, till 2035
- 12.2.1. Molecular Diagnostics Market for Infectious Diseases, till 2035
13. MOLECULAR DIAGNOSTICS MARKET, BY END USERS
- 13.1. Market Movement Analysis
- 13.2. Molecular Diagnostics Market: Distribution by End Users
- 13.2.1. Molecular Diagnostics Market for Laboratories, till 2035
- 13.2.1.1. Molecular Diagnostics Market for Large Laboratories, till 2035
- 13.2.1.2. Molecular Diagnostics Market for Small and Medium-sized Laboratories, till 2035
- 13.2.2. Molecular Diagnostics Market for Hospitals, till 2035
- 13.2.3. Molecular Diagnostics Market for Other End Users, till 2035
- 13.2.1. Molecular Diagnostics Market for Laboratories, till 2035
14. MOLECULAR DIAGNOSTICS MARKET, BY GEOGRAPHICAL REGIONS
- 14.1. Market Movement Analysis
- 14.2. Molecular Diagnostics Market: Distribution by Geographical Regions
- 14.2.1. Molecular Diagnostics Market in North America, till 2035
- 14.2.1.1. Molecular Diagnostics Market in the US, till 2035
- 14.2.1.2. Molecular Diagnostics Market in Canada, till 2035
- 14.2.2. Molecular Diagnostics Market in Europe, till 2035
- 14.2.2.1. Molecular Diagnostics Market in Austria, till 2035
- 14.2.2.2. Molecular Diagnostics Market in Belgium, till 2035
- 14.2.2.3. Molecular Diagnostics Market in France, till 2035
- 14.2.2.4. Molecular Diagnostics Market in Germany, till 2035
- 14.2.2.5. Molecular Diagnostics Market in Italy, till 2035
- 14.2.2.6. Molecular Diagnostics Market in the Netherlands, till 2035
- 14.2.2.7. Molecular Diagnostics Market in Poland, till 2035
- 14.2.2.8. Molecular Diagnostics Market in Spain, till 2035
- 14.2.2.9. Molecular Diagnostics Market in Switzerland, till 2035
- 14.2.2.10. Molecular Diagnostics Market in the UK, till 2035
- 14.2.2.11. Molecular Diagnostics Market in the Rest of Europe, till 2035
- 14.2.3. Molecular Diagnostics Market in Asia, till 2035
- 14.2.3.1. Molecular Diagnostics Market in China, till 2035
- 14.2.3.2. Molecular Diagnostics Market in India, till 2035
- 14.2.3.3. Molecular Diagnostics Market in Indonesia, till 2035
- 14.2.3.4. Molecular Diagnostics Market in Japan, till 2035
- 14.2.3.5. Molecular Diagnostics Market in Singapore, till 2035
- 14.2.3.6. Molecular Diagnostics Market in South Korea, till 2035
- 14.2.3.7. Molecular Diagnostics Market in Thailand, till 2035
- 14.2.3.8. Molecular Diagnostics Market in Rest of Asia, till 2035
- 14.2.4. Molecular Diagnostics Market in Latin America, till 2035
- 14.2.4.1. Molecular Diagnostics Market in Brazil, till 2035
- 14.2.4.2. Molecular Diagnostics Market in Argentina, till 2035
- 14.2.4.3. Molecular Diagnostics Market in Mexico, till 2035
- 14.2.4.4. Molecular Diagnostics Market in Rest of Latin America, till 2035
- 14.2.5. Molecular Diagnostics Market in Middle East and North Africa, till 2035
- 14.2.5.1. Molecular Diagnostics Market in Egypt, till 2035
- 14.2.5.2. Molecular Diagnostics Market in Israel, till 2035
- 14.2.5.3. Molecular Diagnostics Market in Saudi Arabia, till 2035
- 14.2.5.4. Molecular Diagnostics Market in the Rest of Middle East and North Africa, till 2035
- 14.2.6. Molecular Diagnostics Market in Rest of the World, till 2035
- 14.2.6.1. Molecular Diagnostics Market in Australia, till 2035
- 14.2.6.2. Molecular Diagnostics Market in New Zealand, till 2035
- 14.2.1. Molecular Diagnostics Market in North America, till 2035
15. MOLECULAR DIAGNOSTICS MARKET, BY LEADING PLAYERS
- 15.1. Molecular Diagnostics Market: Distribution of Leading Players by Annual Revenues
16. MARKET OVERVIEW: LEADING MOLECULAR DIAGNOSTIC SOLUTION PROVIDERS
- 16.1. Molecular Diagnostic Solution: Overall Market Landscape
- 16.1.1. Analysis by Type of Technology
- 16.1.2. Analysis by Diagnostic Applications
- 16.1.3. Analysis by Type of Technology and Diagnostic Applications
- 16.2. Molecular Diagnostics: Solution Providers Landscape
- 16.2.1. Analysis by Year of Establishment
- 16.2.2. Analysis by Company Size
- 16.2.3. Analysis by Location of Headquarters
- 16.2.4. Analysis by Company Ownership
17. COMPANY COMPETITIVENESS ANALYSIS: MOLECULAR DIAGNOSTIC SOLUTION PROVIDERS
- 17.1. Methodology and Key Parameters Assessed
- 17.2. Molecular Diagnostic Solution Providers: Competitiveness Analysis of Very Large Players
- 17.3. Molecular Diagnostic Solution Providers: Competitiveness Analysis of Large Players
- 17.4. Benchmarking Analysis: Leading Molecular Diagnostics Solution Providers
- 17.4.1. Benchmarking of Companies
- 17.4.1.1. Roche: Benchmarking Analysis
- 17.4.1.2. Abbott: Benchmarking Analysis
- 17.4.1.3. Thermo Fisher Scientific: Benchmarking Analysis
- 17.4.1.4. Qiagen: Benchmarking Analysis
- 17.4.1.5. bioMerieux: Benchmarking Analysis
- 17.4.1.6. DiaSorin: Benchmarking Analysis
- 17.4.1.7. Illumina: Benchmarking Analysis
- 17.4.1.8. Sysmex: Benchmarking Analysis
- 17.4.1.9. Perkin Elmer: Benchmarking Analysis
- 17.4.1.10. Bio-Rad: Benchmarking Analysis
- 17.4.2. Benchmarking of Parameters
- 17.4.2.1. Leading Molecular Diagnostics Solution Providers: Benchmarking by Competitiveness
- 17.4.2.2. Leading Molecular Diagnostic Solution Providers: Benchmarking by Type of Technology Score
- 17.4.2.3. Leading Molecular Diagnostic Solution Providers: Benchmarking by Diagnostic Applications Score
- 17.4.1. Benchmarking of Companies
18. COMPANY PROFILES: MOLECULAR DIAGNOSTICS SOLUTION PROVIDERS BASED IN NORTH AMERICA
- 18.1. Detailed Company Profiles
- 18.1.1. Abbott
- 18.1.1.1. Company Overview
- 18.1.1.2. Product Portfolio
- 18.1.1.3. Financial Information
- 18.1.1.4. Recent Developments and Future Outlook
- 18.1.2. Agilent Technologies
- 18.1.3. BD
- 18.1.4. Danaher
- 18.1.5. Thermo Fisher Scientific
- 18.1.1. Abbott
- 18.2. Short Company Profiles
- 18.2.1. Bio-Rad
- 18.2.1.1. Company Overview
- 18.2.1.2. Product Portfolio
- 18.2.2. Illumina
- 18.2.3. Hologic
- 18.2.4. PerkinElmer
- 18.2.5. QuidelOrtho
- 18.2.1. Bio-Rad
19. COMPANY PROFILES: MOLECULAR DIAGNOSTICS SOLUTION PROVIDERS BASED IN EUROPE
- 19.1. Detailed Company Profiles
- 19.1.1. bioMerieux
- 19.1.1.1. Company Overview
- 19.1.1.2. Product Portfolio
- 19.1.1.3. Financial Information
- 19.1.1.4. Recent Developments and Future Outlook
- 19.1.2. Grifols
- 19.1.3. Roche
- 19.1.4. Siemens Healthineers
- 19.1.1. bioMerieux
- 19.2. Brief Company Profiles
- 19.2.1. DiaSorin
- 19.2.1.1. Company Overview
- 19.2.1.2. Product Portfolio
- 19.2.2. Qiagen
- 19.2.1. DiaSorin
20. COMPANY PROFILES: MOLECULAR DIAGNOSTICS SOLUTION PROVIDERS BASED IN ASIA
- 20.1. Detailed Company Profiles
- 20.1.1. Sysmex
- 20.1.1.1. Company Overview
- 20.1.1.2. Product Portfolio
- 20.1.1.3. Financial Information
- 20.1.1.4. Recent Developments and Future Outlook
- 20.1.1. Sysmex
- 20.2. Brief Company Profiles
- 20.2.1. BGI Genomics
- 20.2.1.1. Company Overview
- 20.2.1.2. Product Portfolio
- 20.2.2. Sansure
- 20.2.3. Seegene
- 20.2.1. BGI Genomics
21. PORTER'S FIVE FORCES ANALYSIS
- 21.1. Methodology and Assumptions
- 21.2. Key Parameters
- 21.2.1. Threats of New Entrants
- 21.2.2. Bargaining Power of Buyers
- 21.2.3. Bargaining Power of Suppliers
- 21.2.4. Threats of Substitute Products
- 21.2.5. Rivalry among Existing Competitors
- 21.3. Porter's Five Force Analysis: Harvey Ball Analysis
- 21.4. Concluding Remarks



















