
|
시장보고서
상품코드
1817404
아데노연관바이러스(AAV) 벡터 시장 : 업계 동향과 세계 예측 - 치료 유형별, 치료 영역별, 투여 경로별, 사업 규모별, 지역별Adeno-Associated Viral (AAV) Vector Market: Industry Trends and Global Forecasts - Distribution by Type of Therapy, Therapeutic Area, Route of Administration, Scale of Operation and Geographical Regions |
||||||
세계 아데노연관바이러스(AAV) 벡터 시장 : 개요
세계의 AAV 벡터 시장 규모는 현재로서는 36억 달러, 2035년에는 60억 달러에 달할 것으로 추정되며, 예측 기간 중 CAGR은 5.3%에 달할 전망입니다.
시장 세분화에서는 시장 규모 및 기회 분석을 다음과 같은 매개 변수로 구분합니다.
치료 유형
- 유전자 강화 요법
- 유전자 조절 요법
치료 영역
- 암 질환
- 희귀질환
- 신경질환
- 대사성 질환
- 근골격계 질환
- 피부과 질환
- 감염성 질환
- 심혈관 질환
- 유전질환
- 안과 질환
- 기타
투여 경로
- 정맥 투여
- 망막하 경로
- 유리체내 투여
- 기타
사업 규모
- 전임상
- 임상
- 상업
주요 지역
- 북미
- 유럽
- 아시아태평양
- 중동 및 북아프리카
- 기타
세계 AAV 시장 성장 및 동향
최근 수년간 유전자 치료는 세포 수준에서 질병의 근본 원인을 표적으로 삼을 수 있다는 점에서 수요가 급증하고 있습니다. 현재 2,000개 이상의 유전자 치료제가 다양한 임상 개발 단계에서 평가되고 있습니다. 이러한 치료법에 대한 관심 증가는 새로운 전달 벡터에 대한 수요 증가로 이어졌습니다. AAV 벡터는 사용 가능한 다양한 유전자 도입 벡터 중 가장 효율적인 바이러스 벡터로 부상하고 있습니다. 현재 6가지 AAV 기반 유전자 치료제가 다양한 적응증으로 승인되었습니다. 현재 시장 역학에 따르면 전 세계 290여개 기업이 수요 증가에 대응하기 위해 AAV 벡터 기반 치료제를 개발하고 있습니다.
AAV 기술은 50년 이상의 역사를 가지고 있으며, 지속적으로 발전하고 있으며, 유전자 치료 목적으로 가장 널리 사용되는 유전자 도입 시스템 중 하나가 되었습니다. 또한 AAV 벡터는 구조가 단순하고 질병과의 연관성이 없기 때문에 많은 의료 용도에서 가장 선호되는 벡터입니다. 최근 AAV 벡터 시장은 더 큰 유전 물질 전달을 가능하게 하는 다양한 바이러스 및 비바이러스 시스템과의 경쟁에 직면하고 있습니다. 더 큰 복잡성은 GMP AAV 생산 공정의 스케일업에 따른 어려움에서 비롯됩니다.
AAV 바이러스 벡터 분야의 현재 동향과 예상되는 기회를 고려할 때, 이 분야는 가까운 미래에 괄목할 만한 성장을 이룰 것으로 보입니다.
세계 AAV 시장 주요 인사이트
이 보고서는 세계 AAV 바이러스 벡터 시장의 현황을 조사하고 업계의 잠재적인 성장 기회를 파악합니다. 이 보고서의 주요 조사 결과는 다음과 같습니다.
- 아데노연관바이러스 벡터를 기반으로 한 635개의 치료법이 이 분야의 이해관계자들에 의해 다양한 질환의 적응증으로 평가되고 있습니다.
- 대부분의 치료제(42%)가 전임상 단계에 있으며, 그 다음으로 임상 단계에 있는 치료제(30%)가 많습니다. 현재 대부분의 치료법은 여러 질병을 치료하기 위해 유전자 강화 접근법을 사용하고 있습니다.
- 현재 전 세계 약 95개 기업이 AAV 치료제 개발을 지원하기 위해 다양한 규모의 서비스를 제공한다고 주장하고 있습니다.
- 여러 이해관계자들이 아데노연관바이러스 벡터 기반 치료법을 개발하기 위해 전 세계에서 많은 노력을 기울이고 있습니다.
- 현재 55개의 아데노연관바이러스 벡터 기술/플랫폼이 시장에 나와 있으며, 이는 AAV 치료에 대한 수요 증가에 대응하기 위해 다양한 제조업체에서 사용할 수 있습니다.
- 이 분야의 기업이 개발한 기술/플랫폼은 모두 아데노연관바이러스 벡터 제조에 특화된 기술/플랫폼이며, 대부분 신경질환을 대상으로 개발되고 있습니다.
- 임상시험의 대부분(65%)은 지난 3년간 등록되었으며, 상당수의 임상시험은 현재 초기 단계(임상 1상 및 임상 2상)에 있습니다.
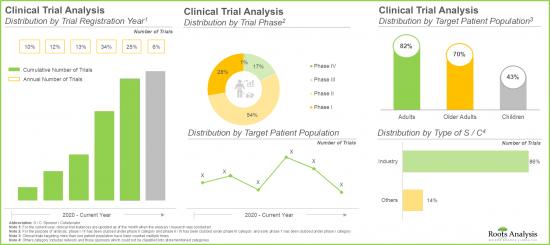
- 인수합병(M&A)(22%)는 새로운 시장 진입과 제품 포트폴리오 확장을 위해 업계 이해관계자들이 선호하는 파트너십 모델 유형으로 부상했습니다.
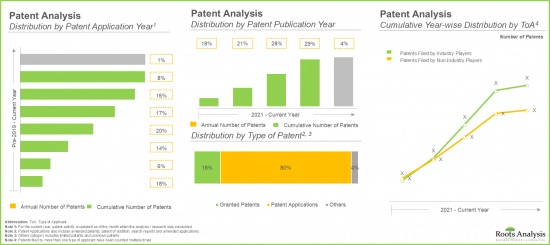
- 2021년 이후 아데노연관바이러스 벡터 분야에서 1,600건 이상의 특허가 공개되었으며, 이 중 60% 이상이 지난 5년간 출원된 특허입니다.
- 시판된 치료제나 후기 단계의 치료제에서 창출되는 매출 측면에서 향후 기회는 다양한 치료 질환, 치료법 유형, 투여 경로에 잘 분산되어 있을 것으로 예측됩니다.
- AAV 제조 기술은 높은 표적 특이성, 광범위한 조직 지향성 등 다양한 이점을 제공하므로 예측 기간 중 시장은 연평균 7.7%의 성장률을 보일 것으로 보입니다.
세계 AAV 시장 주요 부문
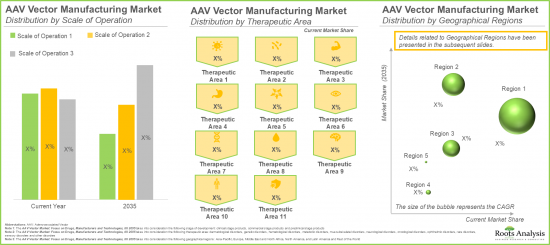
치료 분야별로 보면 시장은 근육 관련 질환, 유전성 질환, 혈액 질환, 안과 질환, 피부 질환, 대사성 질환으로 구분됩니다. 특히 뒤쉔 근이영양증(DMD), 척수성 근위축증과 같은 중증 근육질환 치료에 AAV 벡터를 이용한 치료법 채택이 증가하고 있기 때문입니다.
치료 유형별로 보면 세계 AAV 벡터 기반 치료제 시장은 유전자 강화 요법과 유전자 조절 요법으로 구분됩니다. 올해에는 유전자 강화 치료의 매출이 AAV 벡터 기반 치료 산업을 완전히 주도하고 있습니다. 또한 유전자 조절 요법의 점유율이 크게 증가하여 예측 기간 중 CAGR 61%를 보일 것으로 예측됩니다.
투여 경로 측면에서 AAV 벡터 기반 치료 산업은 정맥내 경로, 망막하 경로, 초자체내 경로, 기타 경로로 구분됩니다. 현재 정맥 투여 경로가 가장 높은 시장 점유율을 차지하고 있는 이유는 정맥 투여 경로가 전신에 신속하고 균일하게 치료제를 투여할 수 있기 때문입니다. 또한 초자체내 경로로 투여되는 치료제의 점유율이 크게 증가하여 예측 기간 중 CAGR 64%를 보일 것으로 예측됩니다. 이는 안과 질환에 대한 AAV 기반 치료 승인 건수가 증가하고 있으며, 그 주요 경로로 초자체내 경로가 부상하고 있는 결과입니다.
이 부문에서는 북미, 유럽, 아시아태평양, 라틴아메리카 및 기타 지역의 AAV 벡터 기반 치료제 시장의 분포에 초점을 맞추었습니다. 기본 추정에 따르면 북미가 올해 AAV 벡터 기반 치료제 시장 점유율의 대부분(75%)을 차지할 가능성이 높습니다. 이는 이 지역에 다수의 임상시험을 실시할 수 있는 첨단 의료 인프라가 구축되어 있기 때문입니다.
AAV 시장 진출기업 사례
- Astellas Pharma
- Charles River Laboratories
- Lonza
- Patheon pharma services
- Sanofi
- WuXi AppTec
- Sarepta Therapeutics
- Uniqure
- Spark Therapeutics
- PTC Therapeutics
- Biomarin Pharmaceutical
- Novartis
세계의 AAV 시장 조사 대상
- 시장 규모 및 기회 분석 : 이 보고서는 세계 AAV 벡터 시장을 상세하게 분석하고,(A) 치료 유형,(B) 치료 분야,(C) 투여 경로,(D) 사업 규모,(E) 지역 등 주요 시장 부문에 초점을 맞추었습니다.
- 아데노연관바이러스 벡터 제조 시장 현황:(A)개발 현황,(B)치료 영역,(C)표적 유전자/분자 유형,(D)치료 유형,(E)사용하는 유전자 도입 방법,(F)투여 경로,(G)특별 지정,(H)설립 연도,(I)기업 규모,(J)본사 소재지 등 주요 시장 부문에 초점을 맞추어 세계 AAV 벡터 시장을 상세하게 분석합니다.
- 아데노연관바이러스 벡터 제조 시장의 기술/플랫폼 현황: A) 제조되는 벡터의 유형, B) 제조되는 바이러스 벡터의 유형, C) 사업 규모, D) 용도, E) 치료분야, F) 설립연도, G) 기업규모, H) 본사 소재지 등 다양한 매개변수를 고려하여 아데노연관바이러스 벡터 제조 기술/플랫폼에 대한 종합적인 평가.
- 경쟁 분석 : AAV 바이러스 벡터 제조업체의 종합적인 경쟁 분석으로(A) 제조업체의 강점,(B) 제품 포트폴리오의 강점 등의 요인을 검증합니다.
- 기술 경쟁 분석 : A) 공급업체 역량,(B) 주요 기술 사양 등의 요인을 검증하여 AAV 바이러스 벡터 제조 기술에 대한 종합적인 경쟁 분석을 실시합니다.
- 기업 개요: A) 기업 개요, B) 재무 정보(가능한 경우), C) 의약품 포트폴리오, D) 최근 동향 및 미래 전망에 초점을 맞춘 진출기업의 상세한 기업 개요 및 의약품 프로파일.
- 임상시험 분석 :(A) 임상시험 등록 연도,(B) 현재 상황,(C) 개발 단계,(D) 등록 환자 수,(E) 스폰서/공동연구자 유형,(F) 임상시험의 지역 분포 등 여러 매개변수를 기반으로 한 임상시험에 대한 인사이트 분석.
- 파트너십 및 공동연구: A)제휴 연도,(B)제휴 유형,(C)치료 분야,(D)용도,(E)지역 분포,(F)가장 활발한 진출기업(제휴 수) 등 여러 매개 변수를 기반으로 AAV 벡터 제조 시장의 이해 관계자가 체결한 거래에 대한 상세한 분석.
- 가능성 있는 파트너 분석 : A) 개발자의 강점, B) 제품의 강점, C) 치료 능력, D) 파이프라인의 강점 등 여러 매개변수를 바탕으로 아데노연관바이러스 벡터 및 유전자치료제 제조업체와 파트너십을 맺을 가능성이 있는 기업을 상세하게 검토.
- 특허 분석 :(A) 특허 유형,(B) 공개 연도,(C) 지역 적용성,(D) CPC 기호,(E) 새로운 중점 분야,(F) 업계/비업계 주요 진출기업,(G) 특허 평가 등 다양한 관련 파라미터를 기반으로 AAV 벡터 제조 분야에서 현재까지 출원/부여된 특허를 상세하게 분석합니다. 분석합니다.
- 스타트업 건전성 지수: A) 탐색 단계, 전임상 단계, 임상 단계에 있는 후보물질 수,(B) 특허 수,(C) 파트너십 체결 수 등 관련 파라미터를 기반으로 아데노연관바이러스 벡터 기반 치료제 개발에 종사하는 다양한 스타트업을 종합적으로 분석합니다.
- 아웃소싱: Go/No-Go 프레임워크: 아데노연관바이러스 벡터 제조업체가 자사 제품을 자체 생산할 것인지 CMO에 위탁할 것인지에 대한 의사결정을 용이하게 하기 위해 고려해야 할 다양한 요인에 대한 종합적인 연구.
목차
섹션 1 리포트 개요
제1장 서문
제2장 조사 방법
제3장 시장 역학
- 챕터 개요
- 예측 조사 방법
- 시장 평가 프레임워크
- 예측 툴과 테크닉
- 주요 고려사항
- 제한 사항
제4장 거시경제 지표
- 챕터 개요
- 시장 역학
- 결론
섹션 2 정성적 인사이트
제5장 개요
제6장 서론
- 챕터 개요
- 벡터의 분류
- 아데노연관바이러스 벡터
- 결론
섹션 3 경쟁 구도
제7장 아데노연관바이러스 벡터 요법 : 시장 구도
- 챕터 개요
- 아데노연관바이러스 벡터 기반 치료법 : 시장 구도
- 아데노연관바이러스 벡터 기반 치료법 개발자 : 전체적인 상황
제8장 아데노연관바이러스 벡터 제조업체 : 시장 구도
- 챕터 개요
- 아데노연관바이러스 벡터 제조업체 : 시장 구도
제9장 아데노연관바이러스 벡터 제조 : 기술/플랫폼 상황
- 챕터 개요
- 아데노연관바이러스 벡터 제조 : 기술/플랫폼 상황
- 아데노연관바이러스 벡터 제조 : 기술/플랫폼 프로바이더 상황
제10장 기업 경쟁력 분석 : 아데노연관바이러스 벡터 제조업체
- 챕터 개요
- 전제와 주요 파라미터
- 조사 방법
- 피어 그룹의 개요
- 기업 경쟁력 분석
제11장 기술 경쟁력 분석
- 챕터 개요
- 전제와 주요 파라미터
- 조사 방법
- 피어 그룹의 개요
- 아데노연관바이러스 벡터 기술/플랫폼 : 경쟁력 분석
섹션 4 기업 개요
제12장 시판약 개요
- 챕터 개요
- Elevidys(Sarepta Therapeutics 개발)
- Hemgenix(Uniqure 개발)
- Kebilidi(PTC Therapeutics 개발)
- Luxturna(Spark Therapeutics 개발)
- Roctavian(Biomarin Pharmaceutical 개발)
- Zolgensma(Novartis 개발)
제13장 기업 개요 : 주요 아데노연관바이러스 벡터 제조업체
- 챕터 개요
- Astellas Pharma
- Charles River Laboratories
- Cytiva
- Lonza
- Patheon Pharma Services
- Sanofi
- Wuxi AppTec
섹션 5 시장 동향
제14장 임상시험의 분석
- 챕터 개요
- 범위와 조사 방법
- 아데노연관바이러스 벡터를 이용한 치료법 : 임상시험 분석
제15장 파트너십과 협업
- 챕터 개요
- 파트너십 모델
- 아데노연관바이러스 벡터를 이용한 치료법 개발자 : 파트너십과 협업
- 아데노연관바이러스 벡터 제조업체 : 파트너십과 협업
제16장 파트너 후보 분석
제17장 특허 분석
- 챕터 개요
- 범위와 조사 방법
- 아데노연관바이러스 벡터 시장 : 특허 분석
- 특허 벤치마킹 분석
- 특허 평가
- 인용수 상위 특허
제18장 스타트업 건전성 지표
- 챕터 개요
- 아데노연관바이러스 벡터 기반 치료법을 개발하는 스타트업 기업
- 스타트의업 벤치마킹
제19장 아웃소싱 : GO/NO-GO 프레임워크
- 챕터 개요
- 아웃소싱 : Go/No-Go 프레임워크
- 아데노연관바이러스 벡터 개발 아웃소싱 : Go/No-Go 프레임워크
- 결론
섹션 6 시장 기회 분석
제20장 세계의 아데노연관바이러스 벡터 치료제 시장
제21장 아데노연관바이러스 벡터 기반 치료제 시장(치료 영역별)
제22장 아데노연관바이러스 벡터 기반 치료제 시장(치료 유형별)
제23장 아데노연관바이러스 벡터 기반 치료제 시장(투여 경로별)
제24장 아데노연관바이러스 벡터 기반 치료제 시장(지역별)
제25장 아데노연관바이러스 벡터 기반 치료제 시장 : 치료제의 판매 예측
- 챕터 개요
- 주요 전제와 조사 방법
- 시판되고 있는 아데노수반벡터 기반 치료제 : 판매 예측
- Luxturna
- Hemgenix
- Zolgensma
- Roctavian
- Elevidys
- Upstaza
- EB 101
- BBM H901
- 제III상 아데노수반벡터 기반 치료제 : 판매 예측
- AGTC 501
- Lumevoq
- NFS-01
- RGX-314
- SPK-8011
- Giroctocogene fitelparvovec
- RGX-121
- DTx-401
- DTx-301
- ABO-102
- AAV-RPE65
- Ixoberogene Soroparvovec
- OCU400
제26장 세계의 아데노연관바이러스 벡터 제조 시장
제27장 아데노연관바이러스 벡터 제조 시장(개발 단계별)
제28장 아데노연관바이러스 벡터 제조 시장(치료 영역별)
제29장 아데노연관바이러스 벡터 제조 시장(지역별)
제30장 아데노연관바이러스 벡터 제조 시장(시판, 임상, 전임상 단계 제품별)
섹션 7 기타 독점적 인사이트
제31장 결론
제32장 이그제큐티브 인사이트
섹션 8 부록
제33장 부록 I : 표 데이터
제34장 부록 II : 기업 및 조직 리스트
KSA 25.09.26GLOBAL AAV VECTOR MARKET: OVERVIEW
The global AAV vector market estimated to be 3.6 billion in the current year and USD 6.0 billion by 2035, representing a CAGR of 5.3% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Therapy
- Gene Augmentation Therapies
- Gene Regulation Therapies
Therapeutic Area
- Oncological Disorders
- Rare Disorders
- Neurological Disorders
- Metabolic Disorders
- Musculoskeletal Disorders
- Dermatological Disorders
- Infectious Diseases
- Cardiovascular Disorders
- Genetic Disorders
- Ophthalmic Disorders
- Other Disorders
Route of Administration
- Intravenous Route
- Subretinal Route
- Intravitreal Route
- Other Routes
Scale of Operation
- Preclinical
- Clinical
- Commercial
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and North Africa
- Latin America and Rest of the World
Global AAV Market: Growth and Trends
In the last few years, the demand for gene therapies has surged owing to their potential in targeting the underlying cause of a disease at cellular level. Presently, over 2,000 gene therapies are being evaluated in different phases of clinical development. This increasing interest in such therapies has resulted in an increase in the demand for novel delivery vectors. AAV vectors have emerged the most efficient viral vectors, among the various gene delivery vectors available. In the present year, six AAV based gene therapies have been approved for various indications. The current market dynamics suggest that close to 290 players across the globe are developing AAV vector-based therapies in order to cater to their increasing demand.
The AAV technology has a history spanning over 50 years and is consistently advancing, becoming one of the most widely utilized gene delivery systems for gene therapy purposes. Additionally, due to its uncomplicated structure, and absence of disease association, AAV vector is the most favored vector for numerous medical applications. Lately, the AAV vector market has encountered competition from various viral and non-viral systems that enable the delivery of larger genetic material. Additional complexity arises from the difficulties associated with scaling up GMP AAV production processes.
Given the current trends and anticipated opportunities in the AAV viral vector sector, we anticipate that this field will experience notable growth in the near future.
Global AAV Market: Key Insights
The report delves into the current state of global AAV viral vector market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Close to 635 adeno-associated viral vector-based therapies are being evaluated by stakeholders in this domain for various disease indications.
- Most (42%) therapies are in preclinical stage of development, followed by those in clinical stage (30%); majority of the therapies presently use gene augmentation approach in order to treat several diseases.
- Presently, close to 95 players across the globe claim to offer their services across various scale of operations in order to support the development of AAV therapies; notably, majority of these firms are based in North America.
- Several stakeholders have made significant efforts in order to develop adeno-associated viral vector-based therapies, across the globe; majority of these players have been established post-2010.
- 55 adeno-associated viral vector technologies / platforms are currently available in the market; these can be used by various manufacturers in order to cater the increasing demand for AAV therapies.
- All the technologies / platforms developed by companies engaged in this domain are focused on adeno-associated viral vectors manufacturing; of these, majority are being developed against neurological disorders.
- Majority (65%) of the trials were registered in the last three years; a significant proportion of these trials are currently under early stages of research (phase I and phase II).
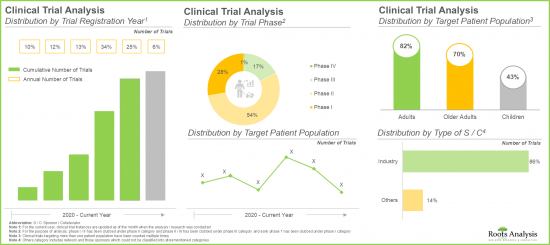
- Mergers and acquisitions (22%) emerged as the preferred type of partnership model adopted by industry stakeholders, as it enables companies to enter new markets and expand their product portfolios.
- Over 1,600 patents have been published in the adeno-associated viral vector domain since 2021; of these, over 60% of the patents have been filed in the last five years.
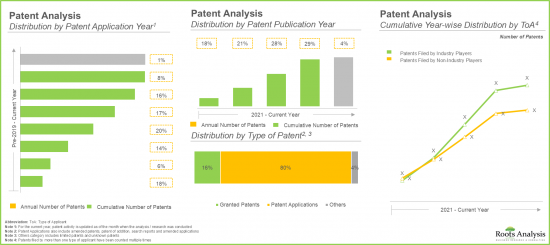
- The future opportunity, in terms of revenues generated from marketed and late-stage therapies, is anticipated to be well distributed across different therapeutic disorders, type of therapy and route of administration.
- The market is likely to witness an annualized growth of 7.7% during the forecast period owing to various benefits offered by AAV manufacturing techniques, including high target specificity and broad tissue tropism.
Global AAV Market: Key Segments
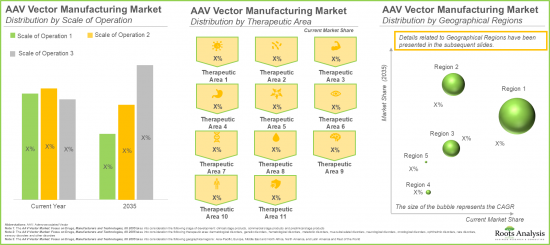
Muscle-related Disorders Segment is Likely to Hold the Largest Market Share
In terms of therapeutic area, the market is segmented across muscle-related disorders, genetic disorders, hematological disorders, ophthalmic disorders, dermatological disorders and metabolic disorders. In the current year, the muscle-related disorders segment occupies the higher AAV vector market share (53%), owing to the increasing adoption of AAV vector-based therapies in the treatment of severe muscle disorders, specifically Duchenne muscular dystrophy (DMD) and spinal muscular atrophy.
Gene Augmentation Therapies Segment Holds the Largest Market Share
In terms of type of therapy, the global AAV vector-based therapies market is segmented across gene augmentation therapies and gene regulation therapies. In the current year, the revenues generated by the sales of gene augmentation therapies completely drive the AAV vector-based therapies industry. Further, the market is likely to witness a considerable increase in the share of gene regulation therapies, growing at a CAGR of 61% during the forecast period.
Intravenous Route Segment Holds the Largest Market Share
In terms of route of administration, the AAV vector based therapies industry is segmented across intravenous route, subretinal route, intravitreal route and other routes. In the current year, intravenous route segment occupies the highest market share due to the ability of intravenous route to deliver therapy quickly and evenly across the entire body. In addition, the market is anticipated to witness a considerable increase in the share of therapies administered via intravitreal route, growing at a CAGR of 64% during the forecast period. This is an outcome of the increasing number of AAV based therapy approvals for ophthalmic disorders, for which intravitreal route has emerged as the primary route.
Europe is likely to Propel in the AAV vector based Therapies Market in the Coming Years
This segment highlights the distribution of AAV vector based therapies market across various geographical regions, namely North America, Europe, Asia-Pacific and Latin America, and rest of the world. Our estimates suggest that North America is likely to capture the majority (~75%) of the AAV vector based therapies market share in the current year. This can be attributed to the availability of advanced healthcare infrastructure within this region to conduct a large number of clinical trials.
Example Players in the AAV Market
- Astellas Pharma
- Charles River Laboratories
- Lonza
- Patheon pharma services
- Sanofi
- WuXi AppTec
- Sarepta Therapeutics
- Uniqure
- Spark Therapeutics
- PTC Therapeutics
- Biomarin Pharmaceutical
- Novartis
Global AAV Market: Research Coverage
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global AAV vector market, focusing on key market segments, including [A] type of therapy, [B] therapeutic area, [C] route of administration, [D] scale of operation and [E] geographical regions.
- Adeno-associated Viral Vector Manufacturing Market Landscape: The report features an in-depth analysis of the global AAV vector market, focusing on key market segments, including [A] status of development, [B] therapeutic area, [C] type of gene / molecule targeted, [D] type of therapy, [E] gene delivery method used, [F] route of administration, [G] special designation awarded, [H] year of establishment, [I] company size, and [J] location of headquarters.
- Adeno-associated Viral Vector Manufacturing Market Technologies / Platforms Landscape: A comprehensive evaluation of adeno-associated viral vector manufacturing technologies / platforms, considering various parameters, such as [A] type of vector manufactured, [B] type of viral vector manufactured, [C] scale of operation [D] application area [E] therapeutic area, [F] year of establishment, [G] company size, and [H] location of headquarters .
- Company Competitiveness Analysis: A comprehensive competitive analysis of AAV viral vector manufacturers, examining factors, such as [A] manufacturer strength and [B] product portfolio strength.
- Technology Competitiveness Analysis: A comprehensive competitive analysis of AAV viral vector manufacturing technologies, examining factors, such as [A] supplier power and [B] key technology specifications.
- Company Profiles: In-depth company and drug profiles of the players, focusing on [A] company overview, [B] financial information (if available), [C] drug portfolio and [D] recent developments and an informed future outlook.
- Clinical Trial Analysis: An insightful analysis of clinical studies, based on several parameters, such as [A] trial registration year, [B] current status, [C] phase of development, [D], enrolled patient population [E] type of sponsor / collaborator and [F] regional distribution of trials.
- Partnerships and Collaborations: An in-depth analysis of the deals inked by stakeholders in the AAV vector manufacturing market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] therapeutic area, [D] application area, [E] geographical distribution [F] most active players (in terms of number of partnerships).
- Likely Partners Analysis: A detailed review of the companies with the likelihood of establishing partnerships with adeno-associated viral vector and gene therapy product manufacturers, based on several parameters, such as [A] developer strength, [B] product strength, [C] therapeutic capability and [D] pipeline strength.
- Patent Analysis: An in-depth analysis of patents filed / granted till date in the AAV vector manufacturing domain, based on various relevant parameters, such as [A] type of patent, [B] publication year, [C] regional applicability, [D] CPC symbols, [E] emerging focus areas, [F] leading industry / non-industry players and [G] patent valuation.
- Start-up Health Indexing: A comprehensive analysis of the various start-ups engaged in the development of adeno-associated viral vectors-based therapies, based on relevant parameters, such as [A] number of candidates in discovery, preclinical and clinical phases of development, [B] number of patents and [C] number of partnerships established.
- Outsourcing: Go / No-Go Framework: An exhaustive study of the various factors that need to be taken into consideration by adeno-associated viral vector manufacturers to facilitate decision making to manufacture their respective products in-house or engage the services of a CMO.
Key Questions Answered in this Report
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
Reasons to Buy this Report
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
Additional Benefits
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
SECTION I: REPORT OVERVIEW
1. PREFACE
- 1.1. Introduction
- 1.2. Market Share Insights
- 1.3. Key Market Insights
- 1.4. Report Coverage
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.2.1. Market Landscape and Market Trends
- 2.2.2. Market Forecast and Opportunity Analysis
- 2.2.3. Comparative Analysis
- 2.3. Database Building
- 2.3.1. Data Collection
- 2.3.2. Data Validation
- 2.3.3. Data Analysis
- 2.4. Project Methodology
- 2.4.1. Secondary Research
- 2.4.1.1. Annual Reports
- 2.4.1.2. Academic Research Papers
- 2.4.1.3. Company Websites
- 2.4.1.4. Investor Presentations
- 2.4.1.5. Regulatory Filings
- 2.4.1.6. White Papers
- 2.4.1.7. Industry Publications
- 2.4.1.8. Conferences and Seminars
- 2.4.1.9. Government Portals
- 2.4.1.10. Media and Press Releases
- 2.4.1.11. Newsletters
- 2.4.1.12. Industry Databases
- 2.4.1.13. Roots Proprietary Databases
- 2.4.1.14. Paid Databases and Sources
- 2.4.1.15. Social Media Portals
- 2.4.1.16. Other Secondary Sources
- 2.4.2. Primary Research
- 2.4.2.1. Types of Primary Research
- 2.4.2.1.1. Qualitative Research
- 2.4.2.1.2. Quantitative Research
- 2.4.2.1.3. Hybrid Approach
- 2.4.2.2. Advantages of Primary Research
- 2.4.2.3. Techniques for Primary Research
- 2.4.2.3.1. Interviews
- 2.4.2.3.2. Surveys
- 2.4.2.3.3. Focus Groups
- 2.4.2.3.4. Observational Research
- 2.4.2.3.5. Social Media Interactions
- 2.4.2.4. Key Opinion Leaders Considered in Primary Research
- 2.4.2.4.1. Company Executives (CXOs)
- 2.4.2.4.2. Board of Directors
- 2.4.2.4.3. Company Presidents and Vice Presidents
- 2.4.2.4.4. Research and Development Heads
- 2.4.2.4.5. Technical Experts
- 2.4.2.4.6. Subject Matter Experts
- 2.4.2.4.7. Scientists
- 2.4.2.4.8. Doctors and Other Healthcare Providers
- 2.4.2.5. Ethics and Integrity
- 2.4.2.5.1. Research Ethics
- 2.4.2.5.2. Data Integrity
- 2.4.2.1. Types of Primary Research
- 2.4.3. Analytical Tools and Databases
- 2.4.1. Secondary Research
- 2.5. Robust Quality Control
3. MARKET DYNAMICS
- 3.1. Chapter Overview
- 3.2. Forecast Methodology
- 3.2.1. Top-down Approach
- 3.2.2. Bottom-up Approach
- 3.2.3. Hybrid Approach
- 3.3. Market Assessment Framework
- 3.3.1. Total Addressable Market (TAM)
- 3.3.2. Serviceable Addressable Market (SAM)
- 3.3.3. Serviceable Obtainable Market (SOM)
- 3.3.4. Currently Acquired Market (CAM)
- 3.4. Forecasting Tools and Techniques
- 3.4.1. Qualitative Forecasting
- 3.4.2. Correlation
- 3.4.3. Regression
- 3.4.4. Extrapolation
- 3.4.5. Convergence
- 3.4.6. Sensitivity Analysis
- 3.4.7. Scenario Planning
- 3.4.8. Data Visualization
- 3.4.9. Time Series Analysis
- 3.4.10. Forecast Error Analysis
- 3.5. Key Considerations
- 3.5.1. Demographics
- 3.5.2. Government Regulations
- 3.5.3. Reimbursement Scenarios
- 3.5.4. Market Access
- 3.5.5. Supply Chain
- 3.5.6. Industry Consolidation
- 3.5.7. Pandemic / Unforeseen Disruptions Impact
- 3.6. Limitations
4. MACRO-ECONOMIC INDICATORS
- 4.1. Chapter Overview
- 4.2. Market Dynamics
- 4.2.1. Time Period
- 4.2.1.1. Historical Trends
- 4.2.1.2. Current and Forecasted Estimates
- 4.2.2. Currency Coverage
- 4.2.2.1. Major Currencies Affecting the Market
- 4.2.2.2. Factors Affecting Currency Fluctuations
- 4.2.2.3. Impact of Currency Fluctuations on the Industry
- 4.2.3. Foreign Currency Exchange Rate
- 4.2.3.1. Impact of Foreign Exchange Rate Volatility on the Market
- 4.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 4.2.4. Recession
- 4.2.4.1. Assessment of Current Economic Conditions and Potential Impact on the Market
- 4.2.4.2. Historical Analysis of Past Recessions and Lessons Learnt
- 4.2.5. Inflation
- 4.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 4.2.5.2. Potential Impact of Inflation on the Market Evolution
- 4.2.6. Interest Rates
- 4.2.6.1. Interest Rates and Their Impact on the Market
- 4.2.6.2. Strategies for Managing Interest Rate Risk
- 4.2.7. Commodity Flow Analysis
- 4.2.7.1. Type of Commodity
- 4.2.7.2. Origins and Destinations
- 4.2.7.3. Values and Weights
- 4.2.7.4. Modes of Transportation
- 4.2.8. Global Trade Dynamics
- 4.2.8.1. Import Scenario
- 4.2.8.2. Export Scenario
- 4.2.8.3. Trade Policies
- 4.2.8.4. Strategies for Mitigating the Risks Associated with Trade Barriers
- 4.2.8.5. Impact of Trade Barriers on the Market
- 4.2.9. War Impact Analysis
- 4.2.9.1. Russian-Ukraine War
- 4.2.9.2. Israel-Hamas War
- 4.2.10. COVID Impact / Related Factors
- 4.2.10.1. Global Economic Impact
- 4.2.10.2. Industry-specific Impact
- 4.2.10.3 .Government Response and Stimulus Measures
- 4.2.10.4. Future Outlook and Adaptation Strategies
- 4.2.11. Other Indicators
- 4.2.11.1. Fiscal Policy
- 4.2.11.2. Consumer Spending
- 4.2.11.3. Gross Domestic Product
- 4.2.11.4. Employment
- 4.2.11.5. Taxes
- 4.2.11.6. Stock Market Performance
- 4.2.11.7. Cross Border Dynamics
- 4.2.1. Time Period
- 4.3. Conclusion
SECTION II: QUALITATIVE INSIGHTS
5. EXECUTIVE SUMMARY
- 5.1. Executive Summary: Market Landscape
- 5.2. Executive Summary: Market Trends
- 5.3. Executive Summary: Market Forecast and Opportunity Analysis
6. INTRODUCTION
- 6.1. Chapter Overview
- 6.2. Classification of Vectors
- 6.2.1. Viral Vectors
- 6.2.1.1. Retrovirus Vectors
- 6.2.1.2. Lentivirus Vectors
- 6.2.1.3. Adeno-associated Viral Vectors
- 6.2.1.4. Adenovirus Vectors
- 6.2.1.5. Other Viral Vectors
- 6.2.1.5.1. Alphavirus
- 6.2.1.5.2. Foamy Virus
- 6.2.1.5.3. Simian Virus
- 6.2.1.5.4. Vaccinia Virus
- 6.2.1.5.5. Chimeric Viral Vectors
- 6.2.1.5.6. Herpes Simplex Virus
- 6.2.1.5.7. Sendai Virus
- 6.2.2. Non-Viral Vectors
- 6.2.1. Viral Vectors
- 6.3. Adeno-associated Viral Vectors
- 6.3.1. Structure and Design
- 6.3.2. Adeno-associated Viral Vector Life Cycle
- 6.3.3. Application of Adeno-associated Viral Vectors
- 6.3.3.1. Gene Therapy
- 6.3.3.2. Vaccination
- 6.3.4. Advantages of Adeno-associated Viral Vectors
- 6.3.5. Challenges Related to Adeno-associated Viral Vectors
- 6.4. Concluding Remarks
SECTION III: COMPETITIVE LANDSCAPE
7. ADENO-ASSOCIATED VIRAL VECTOR BASED THERAPIES: MARKET LANDSCAPE
- 7.1. Chapter Overview
- 7.2. Adeno-associated Viral Vector Based Therapies: Overall Market Landscape
- 7.2.1. Analysis by Status of Development
- 7.2.2. Analysis by Therapeutic Area
- 7.2.3. Analysis by Status of Development and Therapeutic Area
- 7.2.4. Analysis by Type of Gene / Molecule Targeted
- 7.2.5. Analysis by Type of Therapy
- 7.2.6. Analysis by Type of Gene Delivery Method Used
- 7.2.7. Analysis by Route of Administration
- 7.2.8. Analysis by Special Designation Awarded
- 7.3. Adeno-associated Viral Vector Based Therapy Developers: Overall Landscape
- 7.3.1. Analysis by Year of Establishment
- 7.3.2. Analysis by Company Size
- 7.3.3. Analysis by Location of Headquarters (Region)
- 7.3.4. Analysis by Company Size and Location of Headquarters (Region)
- 7.3.5. Most Active Developers: Analysis by Number of Therapies Developed
8. ADENO-ASSOCIATED VIRAL VECTOR MANUFACTURERS: MARKET LANDSCAPE
- 8.1. Chapter Overview
- 8.2. Adeno-associated Viral Vector Manufacturers: Overall Market Landscape
- 8.2.1. Analysis by Year of Establishment
- 8.2.2. Analysis by Company Size
- 8.2.3. Analysis by Location of Headquarters
- 8.2.4. Analysis by Type of Product Manufactured
- 8.2.5. Analysis by Type of Vector Manufactured
- 8.2.6. Analysis by Scale of Operation
- 8.2.7. Analysis by Type of Manufacturer
- 8.2.8. Analysis by Location of Vector Manufacturing Facility
- 8.2.9. Analysis by Application Area
9. ADENO-ASSOCIATED VIRAL VECTOR MANUFACTURING: TECHNOLOGIES / PLATFORMS LANDSCAPE
- 9.1. Chapter Overview
- 9.2. Adeno-associated Viral Vector Manufacturing: Technologies / Platforms Landscape
- 9.2.1. Analysis by Type of Vector Manufactured
- 9.2.2. Analysis by Type of Viral Vector Manufactured
- 9.2.3. Analysis by Scale of Operation
- 9.2.4. Analysis by Application Area
- 9.2.5. Analysis by Therapeutic Area
- 9.3. Adeno-associated Viral Vector Manufacturing: Technology / Platform Providers Landscape
- 9.3.1. Analysis by Year of Establishment
- 9.3.2. Analysis by Company Size
- 9.3.3. Analysis by Location of Headquarters
- 9.3.4. Analysis by Company Size and Location of Headquarters
- 9.3.5. Most Active Players: Analysis by Number of Technologies / Platforms
10. COMPANY COMPETITIVENESS ANALYSIS: ADENO-ASSOCIATED VIRAL VECTOR MANUFACTURERS
- 10.1. Chapter Overview
- 10.2. Assumptions and Key Parameters
- 10.3. Methodology
- 10.4. Overview of Peer Groups
- 10.5. Company Competitiveness Analysis
- 10.5.1. Adeno-associated viral vector manufacturers headquartered in North America
- 10.5.2. Adeno-associated viral vector manufacturers headquartered in Europe
- 10.5.3. Adeno-associated viral vector manufacturers headquartered in Asia-Pacific
11. TECHNOLOGY COMPETITIVENESS ANALYSIS
- 11.1. Chapter Overview
- 11.2. Assumptions and Key Parameters
- 11.3. Methodology
- 11.4. Overview of Peer Groups
- 11.5. Adeno-associated Viral Vector Technologies / Platforms: Competitiveness Analysis
- 11.5.1. Adeno-associated Viral Vector Technologies / Platforms Provided by Companies Headquartered in North America
- 11.5.2. Adeno-associated Viral Vector Technologies / Platforms Provided by Companies Headquartered in Europe and Asia-Pacific
SECTION IV: COMPANY PROFILES
12. MARKETED DRUG PROFILES
- 12.1. Chapter Overview
- 12.2. Elevidys (Developed by Sarepta Therapeutics)
- 12.2.1. Company Overview
- 12.2.2. Development Timeline
- 12.2.3. Mechanism of Action and Vector Used
- 12.2.4. Manufacturing, Dosage and Sales
- 12.2.5. Target Indication
- 12.2.6. Current Status of Development
- 12.3. Hemgenix (Developed by Uniqure)
- 12.4. Kebilidi (Developed by PTC Therapeutics)
- 12.5. Luxturna (Developed by Spark Therapeutics)
- 12.6. Roctavian (Developed by Biomarin Pharmaceutical)
- 12.7. Zolgensma (Developed by Novartis)
13. COMPANY PROFILES: LEADING ADENO-ASSOCIATED VIRAL VECTOR MANUFACTURERS
- 13.1. Chapter Overview
- 13.2. Astellas Pharma
- 13.2.1. Company Overview
- 13.2.2. Financial Information
- 13.2.3. Vector Manufacturing Related Capabilities
- 13.2.4. Recent Developments and Future Outlook
- 13.3. Charles River Laboratories
- 13.4. Cytiva
- 13.5. Lonza
- 13.6. Patheon Pharma Services
- 13.7. Sanofi
- 13.8. Wuxi AppTec
SECTION V: MARKET TRENDS
14. CLINICAL TRIAL ANALYSIS
- 14.1. Chapter Overview
- 14.2. Scope and Methodology
- 14.3. Adeno-associated Viral Vector Based Therapies: Clinical Trial Analysis
- 14.3.1. Analysis by Trial Registration Year
- 14.3.2. Analysis of Patients Enrolled by Trial Registration Year
- 14.3.3. Analysis by Trial Phase
- 14.3.4. Analysis of Patients Enrolled by Trial Phase
- 14.3.5. Analysis by Trial Status
- 14.3.6. Analysis of Patients Enrolled by Trial Status
- 14.3.7. Analysis by Target Patient Population
- 14.3.8. Analysis by Patient Gender
- 14.3.9. Analysis by Type of Sponsor / Collaborator
- 14.3.10. Analysis by Study Design
- 14.3.10.1. Analysis by Type of Patient Allocation Model Used
- 14.3.10.2. Analysis by Type of Trial Masking Adopted
- 14.3.10.3. Analysis by Type of Trial Intervention Model
- 14.3.10.4. Analysis by Trial Purpose
- 14.3.11. Most Active Players: Analysis by Number of Clinical Trials Sponsored / Collaborated
- 14.3.12. Analysis by Geography
- 14.3.12.1. Analysis of Clinical Trials by Geography
- 14.3.12.2. Analysis of Clinical Trials by Geography and Trial Status
- 14.3.12.3. Analysis of Patients Enrolled by Geography and Trial Status
- 14.3.12.4. Analysis of Clinical Trials by Geography, Trial Status and Trial Phase
15. PARTNERSHIPS AND COLLABORATIONS
- 15.1. Chapter Overview
- 15.2. Partnership Models
- 15.3. Adeno-associated Viral Vector Based Therapy Developers: Partnerships and Collaborations
- 15.3.1. Analysis by Year of Partnership
- 15.3.2. Analysis by Type of Partnership
- 15.3.3. Analysis by Year and Type of Partnership
- 15.3.4. Analysis by Year of Partnership and Type of Partner
- 15.3.5. Analysis by Type of Partnership and Type of Partner
- 15.3.6. Analysis by Therapeutic Area
- 15.3.7. Most Active Players: Analysis by Number of Partnerships
- 15.3.8. Analysis by Geography
- 15.3.8.1. Analysis by Country
- 15.3.8.2. Analysis by Region
- 15.4. Adeno-associated Viral Vector Manufacturers: Partnerships and Collaborations
- 15.4.1. Analysis by Year of Partnership
- 15.4.2. Analysis by Type of Partnership
- 15.4.3. Analysis by Year and Type of Partnership
- 15.4.4. Analysis by Year of Partnership and Type of Partner
- 15.4.5. Analysis by Type of Partnership and Type of Partner
- 15.4.6. Analysis by Therapeutic Area
- 15.4.7. Analysis by Application Area
- 15.4.8. Most Active Players: Analysis by Number of Partnerships
- 15.4.9. Analysis by Geography
- 15.4.9.1. Analysis by Country
- 15.4.9.2. Analysis by Region
16. LIEKLY PARTNER ANALYSIS
- 16.1. Chapter Overview
- 16.2. Adeno-associated Viral Vector Based Therapy Developers: Likely Partners
- 16.2.1. Methodology and Key Parameters
- 16.2.2. Adeno-associated Viral Vector Based Therapy Developers: Likely Partner Analysis
- 16.2.2.1. Most Likely Partners
- 16.2.2.2. Likely Partners
- 16.2.2.3. Less Likely Partners
- 16.2.2.4. Least Likely Partners
17. PATENT ANALYSIS
- 17.1. Chapter Overview
- 17.2. Scope and Methodology
- 17.3. Adeno-associated Viral Vector Market: Patent Analysis
- 17.3.1. Analysis by Patent Application Year
- 17.3.2. Analysis by Patent Publication Year
- 17.3.3. Analysis by Type of Patent and Publication Year
- 17.3.4. Analysis by Patent Jurisdiction
- 17.3.5. Analysis by CPC Symbols
- 17.3.6. Analysis by Type of Applicant
- 17.3.7. Leading Industry Players: Analysis by Number of Patents
- 17.3.8. Leading Non-Industry Players: Analysis by Number of Patents
- 17.3.9. Leading Patent Assignees: Analysis by Number of Patents
- 17.4. Patent Benchmarking Analysis
- 17.4.1. Analysis by Patent Characteristics
- 17.5. Patent Valuation
- 17.6. Leading Patents by Number of Citations
18. START-UP HEALTH INDEXING
- 18.1. Chapter Overview
- 18.2. Start-ups Developing Adeno-associated Viral Vector Based Therapies
- 18.2.1. Analysis by Location of Headquarters
- 18.3. Benchmarking of Start-ups
- 18.3.1. Analysis by Pipeline Strength
- 18.3.2. Analysis by Pipeline Maturity
- 18.3.3. Analysis by Indication Diversity
- 18.3.4. Analysis by Patent Strength
- 18.3.5. Analysis by Partnership Activity
- 18.3.6. Start-ups Health Indexing: Roots Analysis Perspective
19. OUTSOURCING: GO / NO-GO FRAMEWORK
- 19.1. Chapter Overview
- 19.2. Outsourcing: Go / No-Go Framework
- 19.3. Adeno-associated Viral Vector Developers Outsourcing: Go / No-Go Framework
- 19.3.1. Key Assumptions and Parameters
- 19.3.2. Methodology
- 19.3.3. Results and Interpretations
- 19.3.3.1. Adeno-associated Viral Vector Based Therapy Developers: Benchmarking of Small Companies
- 19.3.3.2. Adeno-associated Viral Vector Based Therapy Developers: Benchmarking of Mid-sized Companies
- 19.3.3.3. Adeno-associated Viral Vector Based Therapy Developers: Benchmarking of Large Companies
- 19.4. Concluding Remarks
SECTION VI: MARKET OPPORTUNITY ANALYSIS
20. GLOBAL ADENO-ASSOCIATED VIRAL VECTOR BASED THERAPEUTICS MARKET
- 20.1. Chapter Overview
- 20.2. Assumptions and Methodology
- 20.3. Global Adeno-associated Viral Vector Based Therapeutics Market: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 20.3.1. Scenario Analysis
- 20.3.1.1. Conservative Scenario
- 20.3.1.2. Optimistic Scenario
- 20.3.1. Scenario Analysis
- 20.4. Key Market Segmentations
21. ADENO-ASSOCIATED VIRAL VECTOR BASED THERAPEUTICS MARKET, BY THERAPEUTIC AREA
- 21.1. Chapter Overview
- 21.2. Assumptions and Methodology
- 21.3. Adeno-associated Viral Vector Based Therapeutics Market: Distribution by Therapeutic Area
- 21.3.1. Adeno-associated Viral Vector Based Therapeutics Market for Muscle-related Disorders: Historical Trends (since 2023) and Forecasted Estimates (till 2035)
- 21.3.2. Adeno-associated Viral Vector
Based Therapeutics Market for Genetic Disorders: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 21.3.3. Adeno-associated Viral Vector Based Therapeutics Market for Hematological Disorders: Historical Trends (since 2022) and Forecasted Estimates (till 2035)
- 21.3.4. Adeno-associated Viral Vector Based Therapeutics Market for Ophthalmic Disorders: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 21.3.5. Adeno-associated Viral Vector Based Therapeutics Market for Dermatological Disorders: Forecasted Estimates (till 2035)
- 21.3.6. Adeno-associated Viral Vector Based Therapeutics Market for Metabolic Disorders: Forecasted Estimates (till 2035)
- 21.4. Data Triangulation and Validation
22. ADENO-ASSOCIATED VIRAL VECTOR BASED THERAPEUTICS MARKET, BY TYPE OF THERAPY
- 22.1. Chapter Overview
- 22.2. Key Assumptions and Methodology
- 22.3. Adeno-associated Viral Vector Based Therapeutics Market: Distribution by Type of Therapy
- 22.3.1. Adeno-associated Viral Vector Based Therapeutics Market for Gene Augmentation Therapies, Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 22.3.2. Adeno-associated Viral Vector Based Therapeutics Market for Gene Regulation Therapies, Forecasted Estimates (till 2035)
- 22.4. Data Triangulation and Validation
23. ADENO-ASSOCIATED VIRAL VECTOR BASED THERAPEUTICS MARKET, BY ROUTE OF ADMINISTRATION
- 23.1. Chapter Overview
- 23.2. Key Assumptions and Methodology
- 23.3. Adeno-associated Viral Vector Based Therapeutics Market: Distribution by Route of Administration
- 23.3.1. Adeno-associated Viral Vector Based Therapeutics Market for Intravenous Route: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 23.3.2. Adeno-associated Viral Vector Based Therapeutics Market for Subretinal Route: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 23.3.3. Adeno-associated Viral Vector Based Therapeutics Market for Intravitreal Route: Historical Trends (since 2022) and Forecasted Estimates (till 2035)
- 23.3.4. Adeno-associated Viral Vector Based Therapeutics Market for Other Routes: Historical Trends (since 2022) and Forecasted Estimates (till 2035)
- 23.4. Data Triangulation and Validation
24. ADENO-ASSOCIATED VIRAL VECTOR BASED THERAPEUTICS MARKET, BY GEOGRAPHICAL REGIONS
- 24.1. Chapter Overview
- 24.2. Key Assumptions and Methodology
- 24.3. Adeno-associated Viral Vector Based Therapeutics Market: Distribution by Geographical Regions
- 24.3.1. Adeno-associated Viral Vector Based Therapeutics Market in North America: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 24.3.2. Adeno-associated Viral Vector Based Therapeutics Market in Europe: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 24.3.3. Adeno-associated Viral Vector
Based Therapeutics Market in Asia-Pacific: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 24.3.4. Adeno-associated Viral Vector Based Therapeutics Market in Latin America and Rest of the World: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 24.4. Data Triangulation and Validation
25. ADENO-ASSOCIATED VIRAL VECTOR BASED THERAPEUTICS MARKET: SALES FORECAST OF THERAPIES
- 25.1. Chapter Overview
- 25.2. Key Assumptions and Methodology
- 25.3. Commercialized Adeno-associated Vector Based Therapeutics: Sales Forecast
- 25.3.1. Luxturna
- 25.3.1.1. Sales Forecast
- 25.3.1.2. Net Present Value
- 25.3.1.3. Value Creation Analysis
- 25.3.2. Hemgenix
- 25.3.2.1. Sales Forecast
- 25.3.2.2. Net Present Value
- 25.3.2.3. Value Creation Analysis
- 25.3.3. Zolgensma
- 25.3.3.1. Sales Forecast
- 25.3.3.2. Net Present Value
- 25.3.3.3. Value Creation Analysis
- 25.3.4. Roctavian
- 25.3.4.1. Sales Forecast
- 25.3.4.2. Net Present Value
- 25.3.4.3. Value Creation Analysis
- 25.3.5. Elevidys
- 25.3.5.1. Sales Forecast
- 25.3.5.2. Net Present Value
- 25.3.5.3. Value Creation Analysis
- 25.3.6. Upstaza
- 25.3.6.1. Sales Forecast
- 25.3.6.2. Net Present Value
- 25.3.6.3. Value Creation Analysis
- 25.3.7. EB 101
- 25.3.7.1. Sales Forecast
- 25.3.7.2. Net Present Value
- 25.3.7.3. Value Creation Analysis
- 25.3.8. BBM H901
- 25.3.8.1. Sales Forecast
- 25.3.8.2. Net Present Value
- 25.3.8.3. Value Creation Analysis
- 25.3.1. Luxturna
- 25.4. Phase III Adeno-Associated Vector Based Therapeutics: Sales Forecast
- 25.4.1. AGTC 501
- 25.4.1.1. Sales Forecast
- 25.4.2. Lumevoq
- 25.4.2.1. Sales Forecast
- 25.4.3. NFS-01
- 25.4.3.1. Sales Forecast
- 25.4.4. RGX-314
- 25.4.4.1. Sales Forecast
- 25.4.5. SPK-8011
- 25.4.5.1. Sales Forecast
- 25.4.6. Giroctocogene fitelparvovec
- 25.4.6.1. Sales Forecast
- 25.4.7. RGX-121
- 25.4.7.1. Sales Forecast
- 25.4.7.2. Net Present Value
- 25.4.7.3. Value Creation Analysis
- 25.4.8. DTx-401
- 25.4.8.1. Sales Forecast
- 25.4.9. DTx-301
- 25.4.9.1. Sales Forecast
- 25.4.10. ABO-102
- 25.4.10.1. Sales Forecast
- 25.4.11. AAV-RPE65
- 25.4.11.1. Sales Forecast
- 25.4.12. Ixoberogene Soroparvovec
- 25.4.12.1. Sales Forecast
- 25.4.13. OCU400
- 25.4.13.1. Sales Forecast
- 25.4.1. AGTC 501
26. GLOBAL ADENO-ASSOCIATED VIRAL VECTOR MANUFACTURING MARKET
- 26.1. Chapter Overview
- 26.2. Assumptions and Methodology
- 26.3. Global Adeno-associated Viral Vector Manufacturing Market: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 26.3.1. Scenario Analysis
- 26.3.1.1. Conservative Scenario
- 26.3.1.2. Optimistic Scenario
- 26.3.1. Scenario Analysis
- 26.4. Key Market Segmentations
27. ADENO-ASSOCIATED VIRAL VECTOR MANUFACTURING MARKET, BY STAGE OF DEVELOPMENT
- 27.1. Chapter Overview
- 27.2. Key Assumptions and Methodology
- 27.3. Adeno-associated Viral Vector Manufacturing Market: Distribution by Stage of Development, 2021, 2025 and 2035
- 27.3.1. Adeno-associated Viral Vector Manufacturing Market for Commercial Stage Products: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 27.3.2. Adeno-associated Viral Vector Manufacturing Market for Clinical Stage Products: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 27.3.3. Adeno-associated Viral Vector Manufacturing Market for Preclinical Stage Products: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 27.4. Data Triangulation and Validation
28. ADENO-ASSOCIATED VIRAL VECTOR MANUFACTURING MARKET, BY THERAPEUTIC AREA
- 28.1. Chapter Overview
- 28.2. Key Assumptions and Methodology
- 28.3. Adeno-associated Viral Vector Manufacturing Market: Distribution by Therapeutic Area, 2021, 2025 and 2035
- 28.3.1. Adeno-associated Viral Vector Manufacturing Market for Oncological Disorders: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 28.3.2. Adeno-associated Viral Vector Manufacturing Market for Rare Disorders: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 28.3.3. Adeno-associated Viral Vector Manufacturing Market for Musculoskeletal Disorders: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 28.3.4. Adeno-associated Viral Vector Manufacturing Market for Genetic Disorders: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 28.3.5. Adeno-associated Viral Vector Manufacturing Market for Hematological Disorders: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 28.3.6. Adeno-associated Viral Vector Manufacturing Market for Neurological Disorders: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 28.3.7. Adeno-associated Viral Vector Manufacturing Market for Metabolic Disorders: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 28.3.8. Adeno-associated Viral Vector Manufacturing Market for Sensory Disorders: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 28.3.9. Adeno-associated Viral Vector Manufacturing Market for Ophthalmic Disorders: Historical Trends (since 2021) and Forecasted Estimates (till
2035)
- 28.3.10. Adeno-associated Viral Vector Manufacturing Market for Dermatological Disorders: Historical Trends (since 2025) and Forecasted Estimates (till 2035)
- 28.3.11. Adeno-associated Viral Vector Manufacturing Market for Others: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 28.4. Data Triangulation and Validation
29. ADENO-ASSOCIATED VIRAL VECTOR MANUFACTURING MARKET, BY GEOGRAPHICAL REGIONS
- 29.1. Chapter Overview
- 29.2. Key Assumptions and Methodology
- 29.3. Adeno-associated Viral Vector Manufacturing Market: Distribution by Geographical Regions, 2021, 2025 and 2035
- 29.3.1. Adeno-associated Viral Vector Manufacturing Market in North America: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 29.3.2. Adeno-associated Viral Vector Manufacturing Market in Europe: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 29.3.3. Adeno-associated Viral Vector Manufacturing Market in Asia-Pacific: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 29.3.4. Adeno-associated Viral Vector Manufacturing Market in Middle East and North Africa: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 29.3.5. Adeno-associated Viral Vector Manufacturing Market in Latin America and Rest of the World: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 29.4. Market Dynamics Assessment
- 29.4.1. Penetration Growth (P-G) Matrix
- 29.5. Data Triangulation and Validation
30. ADENO-ASSOCIATED VIRAL VECTOR MANUFACTURING MARKET BY COMMERCIAL, CLINICAL AND PRECLINICAL STAGE PRODUCTS
- 30.1. Chapter Overview
- 30.2. Key Assumptions and Methodology
- 30.3. Key Market Segmentations
- 30.4. Adeno-associated Viral Vector Manufacturing Market for Commercial Stage Products: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 30.4.1. Adeno-associated Viral Vector Manufacturing Market for Commercial Stage Products: Distribution by Therapeutic Area
- 30.4.2. Adeno-associated Viral Vector Manufacturing Market for Commercial Stage Products: Distribution by Route of Administration
- 30.4.3. Adeno-associated Viral Vector Manufacturing Market for Commercial Stage Products: Distribution by Geographical Regions
- 30.5. Adeno-associated Viral Vector Manufacturing Market for Clinical Stage Products: Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 30.5.1. Adeno-associated Viral Vector Manufacturing Market for Clinical Stage Products: Distribution by Phase of Development
- 30.5.2. Adeno-associated Viral Vector Manufacturing Market for Clinical Stage Products: Distribution by Therapeutic
Area
- 30.5.3. Adeno-associated Viral Vector Manufacturing Market for Clinical Stage Products: Distribution by Geographical Regions
- 30.6. Adeno-associated Viral Vector Manufacturing Market for Preclinical Stage Products, Historical Trends (since 2021) and Forecasted Estimates (till 2035)
- 30.6.1. Adeno-associated Viral Vector Manufacturing Market for Preclinical Stage Products: Distribution by Therapeutic Area
- 30.6.2. Adeno-associated Viral Vector Manufacturing Market for Preclinical Stage Products: Distribution by Animal Model Used
- 30.6.3. Adeno-associated Viral Vector Manufacturing Market for Preclinical Stage Products: Distribution by Geographical Regions



















