
|
시장보고서
상품코드
1699286
수탁연구기관 시장 기회, 성장 촉진요인, 산업 동향 분석, 예측(2025-2034년)Contract Research Organization (CRO) Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025-2034 |
||||||
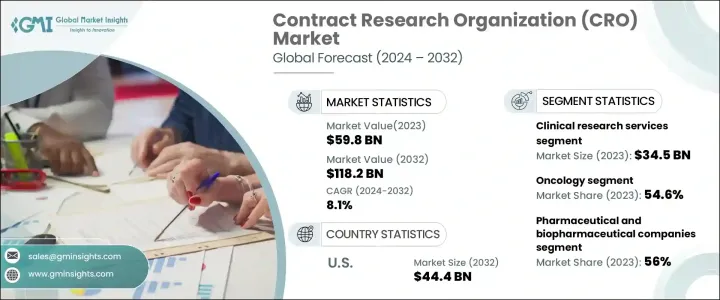
세계 수탁연구기관 시장은 2023년 598억 달러에 달할 것으로 예상되며, 2024년부터 2032년까지 연평균 8.1%의 성장률을 기록할 것으로 전망됩니다.
제약 및 생명공학 기업들이 의약품 개발을 가속화하고 엄격한 규제 요건을 충족하기 위해 비용 효율적인 솔루션을 찾고 있기 때문에 아웃소싱 연구 서비스에 대한 수요가 급증하고 있습니다. 전 세계적으로 임상시험의 수가 증가함에 따라 CRO는 신약개발을 간소화하고, 운영비용을 절감하며, 연구 효율성을 높이는 데 중요한 역할을 하고 있습니다. 의학 연구에 대한 투자는 지속적으로 증가하고 있으며, 시장의 성장 궤도는 더욱 강화되고 있습니다.
만성질환의 증가로 인해 혁신적인 치료법에 대한 필요성이 증가함에 따라 제약회사들은 임상시험, 규제 준수, 실험실 서비스 등의 전문성을 제공하는 CRO에 의존하고 있으며, CRO는 제약 및 생명공학 부문에 없어서는 안 될 존재가 되었습니다. 임상시험 아웃소싱을 통해 기업은 복잡한 규제 환경을 극복하기 위해 CRO의 인프라와 경험을 활용하면서 기술 혁신에 집중할 수 있습니다. 또한, 정밀의료, 유전자 치료, 생물학적 제제의 발전은 연구 아웃소싱 서비스의 범위를 확장하고 업계 관계자들에게 새로운 기회를 창출하고 있습니다. 개인화된 의료 서비스로의 전환은 CRO가 표적 치료 및 첨단 의료 솔루션 개발을 촉진함으로써 전문 연구에 대한 수요를 더욱 증가시키고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 598억 달러 |
| 예상 금액 | 1,182억 달러 |
| CAGR | 8.1% |
서비스 유형별로는 초기 단계 개발, 임상연구, 실험실, 제약 컨설팅 서비스로 구분됩니다. 임상 연구 서비스는 2023년 345억 달러의 매출을 창출하며 업계를 선도했습니다. 이 분야는 혁신적인 치료법과 진단법에 대한 수요가 증가함에 따라 지속적으로 성장하고 있습니다. 임상 연구는 4단계로 나뉘며, 각 단계는 새로운 치료법을 시장에 출시하는 데 필수적입니다. 만성질환 환자의 증가로 인해 임상시험의 필요성이 증가하고 있으며, 제약회사들은 연구 활동의 확장을 추진하고 있습니다. 연구 아웃소싱을 통해 기업은 자원을 최적화하고, 의약품 승인 프로세스를 가속화하며, 임상시험의 효율성을 높일 수 있습니다.
수탁연구기관 시장은 종양학, 심장학, 감염학, 신경학, 소화기학, 간학, 안과학, 기타 등 치료 분야별로 분류되며, 2023년 시장 점유율은 암 분야가 54.6%로 압도적이며, 2032년까지 8.5%의 CAGR을 기록할 것으로 예상됩니다. 암 발병률의 증가로 인해 첨단 치료법 및 의료기기 개발을 위한 연구 노력이 강화되고, 임상시험 활동이 활발해지고 있습니다. 획기적인 치료법을 찾는 움직임은 종양학 연구에 대한 투자를 촉진하고, 연구 위탁 서비스에 대한 수요를 크게 증가시키고 있습니다. 새로운 치료법이 등장하면서 종양학에 특화된 임상시험이 빠르게 확대되고 있으며, 의약품 개발 생태계에서 CRO의 역할이 확고히 자리 잡고 있습니다.
북미 수탁연구기관 시장은 2023년 226억 달러로 평가되며, 2032년에는 444억 달러로 성장할 것으로 예상됩니다. 이 지역에는 주요 제약사 및 생명공학 기업들이 진출하여 시장 점유율 확대에 기여하고 있습니다. 의약품 개발이 복잡해짐에 따라 전문 지식과 첨단 인프라에 대한 요구는 계속 증가하고 있으며, CRO는 종합적인 연구 솔루션, 규제 환경 지원, 데이터 기반 인사이트를 제공함으로써 의학 연구 발전에 기여하고 진화하는 제약 산업에서 중요한 역할을 하고 있습니다.
목차
제1장 조사 방법과 조사 범위
제2장 주요 요약
제3장 업계 인사이트
- 업계 생태계 분석
- 업계에 대한 영향요인
- 성장 촉진요인
- 세계의 연구개발비 증가
- 신흥 국가의 임상시험 수 증가
- 연구개발 활동 아웃소싱 증가
- 기술 진보 상승
- 업계의 잠재적 리스크·과제
- 지적재산권 문제
- 성장 촉진요인
- 성장 가능성 분석
- 임상시험 분석
- 임상시험 건수 : 지역별, 2018-2023년
- 임상시험 건수 : 개발 단계별, 2018-2023년
- 임상시험 건수 : 적응증별, 2018-2023년
- 규제 상황
- 미국
- 유럽
- 아시아태평양
- 싱가포르
- 말레이시아
- 인도네시아
- 태국
- 한국
- 필리핀
- 임상시험-아시아태평양의 우위성
- Drug Discovery & Development 프로세스
- Porters 분석
- PESTEL 분석
제4장 경쟁 구도
- 소개
- 기업 시장 매트릭스 분석
- 경쟁 시장 점유율 분석
- 전략 대시보드
- 인수합병 상황
- CRO 업계의 M&A안건, 2018-2023년
- 완료한 임상시험수탁기관 M&A안건
- CRO M&A의 사모펀드의 영향
- 업계 재편이 시장 전체에 미치는 영향
- 애널리스트의 인사이트
제5장 시장 추정과 예측 : 서비스 유형별, 2021-2032년
- 주요 동향
- 개발 초기 단계 서비스
- 탐색 단계
- 화학·제조·관리(CMC)
- 전임상시험 서비스
- 약물동태/약력학(PK/PD)
- 독성 시험 서비스
- 기타 전임상시험 서비스
- 임상 연구 서비스
- 제I상시험
- 단계 II
- 단계 III
- 단계 IV
- 실험실 서비스
- 바이오 분석 시험 서비스
- 분석 시험 서비스
- 물리적 특성 평가
- 원자재 시험
- 배치 릴리스 시험
- 안정성 시험
- 기타 분석 시험 서비스
- 약사 컨설팅 서비스
제6장 시장 추정과 예측 : 치료 분야별, 2021-2032년
- 주요 동향
- 암영역
- 임상 약리학
- 순환기내과
- 감염증
- 신경학
- 소화기·간 병학
- 안과
- 기타 치료 분야
제7장 시장 추정과 예측 : 최종 용도별, 2021-2032년
- 주요 동향
- 제약·바이오의약품 기업
- 의료기기 기업
- 학술기관
제8장 시장 추정과 예측 : 지역별, 2021-2032년
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 중동 및 아프리카
- 남아프리카공화국
- 사우디아라비아
- 아랍에미리트
제9장 기업 개요
- Caidya
- Charles River Laboratories
- CMIC HOLDINGS
- EPS Holdings
- ICON plc
- IQVIA(Quintiles IMS)
- Iris Pharma
- Laboratory Corporation of America Holdings
- Lexitas Pharma Services
- Medpace Holdings
- Ora
- Parexel International(MA)
- Pharmaceutical Product Development(Thermo Fisher Scientific)
- Premier Research
- ProTrials Research
- Syneos Health
- TFS HealthScience
- The Emmes Company
- Trial Runners
- Veristat
- Worldwide Clinical Trials
- WuXi Clinical(WuXi AppTec)
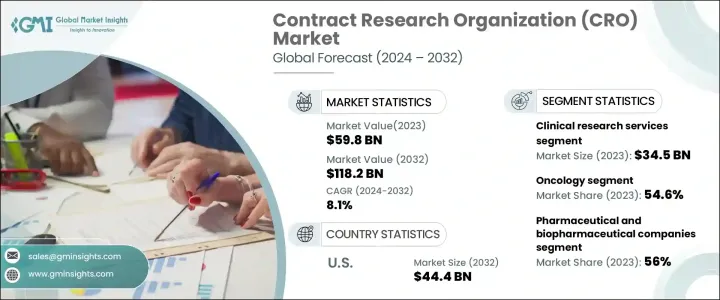
The Global Contract Research Organization Market reached USD 59.8 billion in 2023 and is on track to expand at a CAGR of 8.1% from 2024 to 2032. The demand for outsourced research services is surging as pharmaceutical and biotechnology firms seek cost-effective solutions to accelerate drug development and meet stringent regulatory requirements. With a growing number of clinical trials worldwide, CROs are playing a crucial role in streamlining drug discovery, reducing operational costs, and enhancing research efficiency. Investments in medical research continue to rise, further strengthening the market's growth trajectory.
The increasing prevalence of chronic diseases is fueling the need for innovative treatments, driving pharmaceutical companies to rely on specialized research firms. CROs offer expertise in clinical trials, regulatory compliance, and laboratory services, making them indispensable to the pharmaceutical and biotech sectors. Outsourcing research allows companies to focus on innovation while leveraging the infrastructure and experience of CROs to navigate complex regulatory landscapes. Additionally, advancements in precision medicine, gene therapy, and biologics are expanding the scope of contract research services, creating new opportunities for industry players. The global shift toward personalized healthcare is further amplifying the demand for specialized research, with CROs facilitating the development of targeted therapies and cutting-edge medical solutions.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $59.8 Billion |
| Forecast Value | $118.2 Billion |
| CAGR | 8.1% |
The market is segmented by service type into early phase development, clinical research, laboratory, and regulatory consulting services. Clinical research services led the industry, generating USD 34.5 billion in revenue in 2023. This segment continues to thrive as the demand for innovative treatments and diagnostics rises. Clinical research is structured into four phases, each essential in bringing new therapies to market. The increase in chronic disease cases is intensifying the need for clinical trials, prompting pharmaceutical firms to expand their research efforts. Outsourcing research enables companies to optimize their resources, accelerate drug approval processes, and enhance efficiency in clinical trials.
The contract research organization market is also categorized by therapeutic area, including oncology, cardiology, infectious diseases, neurology, gastroenterology, hepatology, ophthalmology, and others. Oncology dominated the market in 2023 with a commanding 54.6% share and is expected to grow at a CAGR of 8.5% through 2032. The rising incidence of cancer has intensified research efforts to develop advanced therapies and medical devices, leading to an upsurge in clinical trial activity. The push for breakthrough treatments is driving investment in oncology research, leading to a significant increase in demand for contract research services. As new therapies emerge, oncology-focused trials are expanding rapidly, solidifying the role of CROs in the drug development ecosystem.
North America contract research organization market was valued at USD 22.6 billion in 2023, with projections indicating growth to USD 44.4 billion by 2032. The presence of major pharmaceutical and biotechnology firms in the region has contributed to its strong market share. As drug development becomes increasingly complex, the need for specialized expertise and state-of-the-art infrastructure continues to grow. CROs are instrumental in advancing medical research by providing comprehensive research solutions, regulatory support, and data-driven insights, positioning them as key players in the evolving pharmaceutical landscape.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing R&D expenditure worldwide
- 3.2.1.2 Rising number of clinical trials in emerging economies
- 3.2.1.3 Growing outsourcing of R&D activities
- 3.2.1.4 Rising technological advancements
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Intellectual property right issues
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Clinical trial analysis
- 3.4.1 Clinical trial volume, by region, 2018 - 2023
- 3.4.2 Clinical trial volume, by phase of development, 2018 - 2023
- 3.4.3 Clinical trial volume, by indication, 2018 - 2023
- 3.5 Regulatory landscape
- 3.5.1 U.S.
- 3.5.2 Europe
- 3.5.3 Asia Pacific
- 3.5.3.1 Singapore
- 3.5.3.2 Malaysia
- 3.5.3.3 Indonesia
- 3.5.3.4 Thailand
- 3.5.3.5 South Korea
- 3.5.3.6 Philippines
- 3.6 Clinical trials- Asia Pacific advantage
- 3.7 Drug discovery and development process
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2023
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive market share analysis
- 4.4 Strategy dashboard
- 4.5 Merger and acquisition landscape
- 4.5.1 Merger and acquisition deals in the CRO industry, 2018 – 2023
- 4.5.2 Completed contract research organization merger and acquisition deals, 2018 - 2023
- 4.5.3 Influence of private equity in CRO mergers and acquisitions
- 4.5.4 Impact of industry consolidation on the overall market
- 4.5.5 Analyst insights
Chapter 5 Market Estimates and Forecast, By Service Type, 2021 – 2032 ($ Mn)
- 5.1 Key trends
- 5.2 Early phase development services
- 5.2.1 Discovery phase
- 5.2.2 Chemistry, manufacturing and control (CMC)
- 5.2.3 Preclinical services
- 5.2.3.1 Pharmacokinetic/pharmacodynamic (PK/PD)
- 5.2.3.2 Toxicology testing services
- 5.2.3.3 Other preclinical services
- 5.3 Clinical research services
- 5.3.1 Phase I
- 5.3.2 Phase II
- 5.3.3 Phase III
- 5.3.4 Phase IV
- 5.4 Laboratory services
- 5.4.1 Bioanalytical testing services
- 5.4.2 Analytical testing services
- 5.4.2.1 Physical characterization
- 5.4.2.2 Raw material testing
- 5.4.2.3 Batch release testing
- 5.4.2.4 Stability testing
- 5.4.2.5 Other analytical testing services
- 5.5 Regulatory consulting services
Chapter 6 Market Estimates and Forecast, By Therapeutic Area, 2021 – 2032 ($ Mn)
- 6.1 Key trends
- 6.2 Oncology
- 6.3 Clinical pharmacology
- 6.4 Cardiology
- 6.5 Infectious disease
- 6.6 Neurology
- 6.7 Gastroenterology and hepatology
- 6.8 Ophthalmology
- 6.9 Other therapeutic areas
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2032 ($ Mn)
- 7.1 Key trends
- 7.2 Pharmaceutical and biopharmaceutical companies
- 7.3 Medical device companies
- 7.4 Academic institutes
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2032 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Caidya
- 9.2 Charles River Laboratories
- 9.3 CMIC HOLDINGS
- 9.4 EPS Holdings
- 9.5 ICON plc
- 9.6 IQVIA (Quintiles IMS)
- 9.7 Iris Pharma
- 9.8 Laboratory Corporation of America Holdings
- 9.9 Lexitas Pharma Services
- 9.10 Medpace Holdings
- 9.11 Ora
- 9.12 Parexel International (MA)
- 9.13 Pharmaceutical Product Development (Thermo Fisher Scientific)
- 9.14 Premier Research
- 9.15 ProTrials Research
- 9.16 Syneos Health
- 9.17 TFS HealthScience
- 9.18 The Emmes Company
- 9.19 Trial Runners
- 9.20 Veristat
- 9.21 Worldwide Clinical Trials
- 9.22 WuXi Clinical (WuXi AppTec)



















